Full HTML
Techniques, Indications, and Complications of Kidney Biopsy: A Narrative Review
Elmukhtar Habas1, Amnna Rayani2, Kalifa Farfar3, Ala Habas4, Khaled Alarbi5, Aml Habas6, Almehdi Errayes7, Eshrak Habas4, Gamal Alfitori6
Author Affiliation
1 Professor/Senior Consultant, Department of Medicine, Hamad General Hospital, Qatar university, Qatar, Open Libyan University, Libya
2 Professor/Senior Consultant, Tripoli Children Hospital, Open Libyan University, Tripoli-Libya,
3 Consultant, Department of Medicine, Alwakra General Hospital, Qatar,
4 Resident, Department of Medicine, Tripoli Central Hospital, University of Tripoli, Tripoli-Libya,
5 Consultant internal medicine, HGH, Doha-Qatar,
6 Specialist, Tripoli Children Hospital, Open Libyan University, Tripoli-Libya,
7 Senior Consultant, Department of Medicine, Hamad General Hospital, Doha, Qatar.
Abstract
Accurate diagnosis of the cause of acute or chronic kidney dysfunction may require a percutaneous kidney biopsy (PKB). Unfortunately, the invasive nature of the procedure can lead to potential complications that may discourage the KBs. Lack of appropriate communication skills, experienced personnel and equipment, and high procedure costs can negatively impact complication rates and the frequency of conducted KBs. This nonsystematic review assesses KB procedures, indications, contraindications, complications, post-KB monitoring time, and cost. We looked for reviews and original articles published between January 2010 and Jan 2025 on Google, Google Scholar, and PubMed. Different keywords, phrases, and sentences include PKB, renal biopsy, native PKB, ultrasound guide, CT-guided, PKB, allograft PKB, and PKB procedures. KB makes histopathological and immunohistological diagnosis possible, which are necessary for diagnosis and treatment. It is often used despite the known complications. KB and other biochemical assays have tracked transplant rejection and antirejection drugs. Automatic gun spiral needles sizes 14 and 16 capture enough samples with fewer complications than gauge sizes 18 and 20. KB cost is another issue, especially in low-income areas, and deserves additional study.
DOI: 10.63475/yjm.v4i1.0029
Keywords: Percutaneous kidney biopsy (PKB), ultrasound guide PKB, CT-guided PKB, PKB complications, and KB cost
Pages: 51-64
View: 13
Download: 26
DOI URL: https://doi.org/10.63475/yjm.v4i1.0029
Publish Date: 21-05-2025
Full Text
A kidney biopsy (KB) is usually necessary to confirm the clinical diagnosis and to detect the histopathological changes of kidney injury. KB is beneficial in evaluating suspected intrinsic kidney disease, supporting the clinical diagnosis, guiding prognosis and management, and even predicting disease relapse. [1] In the 1950s, KB of the native kidney was introduced into clinical practice, and the technique has since evolved. KB is a reliable procedure that entails taking tissue samples from the kidney, scrutinizing them under a microscope by a pathologist, and doing different immunological investigations.
Persistent hematuria (especially microscopic hematuria) and proteinuria are the usual indications of KB. The golden standard for pathological diagnosis of kidney disease is the KB. [2] Radiologists and nephrologists usually conduct KB, although radiologists have recently been more involved in conducting the KB. [3] In chronic conditions such as diabetes, lupus, and myeloma, particularly in the presence of massive proteinuria, significant hematuria, or progressive kidney dysfunction features, KB helps to assess the extent of kidney involvement. [1]
Percutaneous KB (PKB) is usually taken from the lower pole of the left kidney to avoid major bleeding because this pole has less vascularity. [4] It was started under fluoroscopy guidance [5], and since the 1980s, it has usually been conducted under ultrasound (US) or, infrequently, computer tomography (CT) guidance. [4] Transjugular, open surgical, or laparoscopic KB are conducted in conditions such as simultaneous hepatic/kidney biopsy and severe coagulation diathesis. [6]
Complications during or after a kidney biopsy are rare, and even if they occur, they are usually not severe enough to require major interventions. [7] The most severe complication is bleeding, especially pericapsular hematoma, which can be life-threatening and may compromise kidney function [7]. In rare cases, massive intrarenal bleeding rarely requires embolization. [8] Kidney loss and death are extremely rare. [9] Fortunately, in recent years, the risk of injuring nearby organs and major blood vessels has significantly decreased, providing more confidence in the procedure's safety. Communicating clearly regarding the procedure, indications, and potential complications to the patients and their families is crucial. It is equally vital to offer a safe and supportive environment for the patient, sponsor, and family to share their thoughts and concerns openly. It is also imperative to allow them enough time to thoroughly consider and research further information about the procedure. [10]
In this review, Renal biopsy techniques, indications, costs, and common complications are updated for clinical practitioners and physicians. Furthermore, this review will be a good reference for nephrologists. Furthermore, some knowledge about KB is beneficial and might be needed by the patient and the family. We searched Google, Google Scholar, and PubMed for new reviews and original articles published between Jan 2010 and Jan 2025. Various keywords, phrases, and texts, such as PKB, renal biopsy, native PKB, ultrasound guide PKB, CT-guided PKB, PKB, Kidney biopsy, KB cost, techniques of PKB, and kidney graft biopsy were used. After downloading the appropriate articles, they were distributed according to this review subtitled between the authors. Each author read the assigned articles and summarized them. After several discussion meetings, we assigned the three principal authors to structure and write the current article in its final form.
Although KB is an integral practice in clinical medicine for renal disease diagnosis, its recommendations have no harmony. [11] KB is commonly utilized to ascertain the exact diagnosis in acute or chronic glomerulonephritis, renal parenchymal disease, or perplexing renal function abnormalities. Limited research has demonstrated that implementing automated biopsy needles and real-time ultrasound guidance has improved the success rate of diagnoses by more than 95% in cases involving more recent technologies. [12] The principal purpose of CT-guided biopsy was to facilitate the detection of malignant disease; [13] however, recently, it has also been used to diagnose glomerular and kidney parenchymal diseases. The main indications of KB include nephrotic syndrome, lupus nephritis when presenting with nephrotic syndrome, and glomerulonephritis types. As in primary and secondary amyloidosis, KB is also indicated in massive proteinuria, when histology might affect therapy plans. Relapsed nephrotic syndrome, either in adults or children is an indication for KB. It is well known that malignancy diseases are associated with kidney involvement, and membranous nephropathy, especially in solid tumors. However, it can occur in other malignancies such as lymphoma and leukemia. [14,15] In malignancies, KB is rarely indicated because the associated nephrotic syndrome usually improves with malignancy therapy and cure. [16] Some obese individuals may develop proteinuria in the nephrotic range, which requires KB to exclude focal segmental glomerulosclerosis (FSGS). A KB is also necessary if individuals with proteinuria have positive serum anti-phospholipase A2 receptor (PLA2R) antibodies, evidence of hepatitis B or positive antinuclear antibodies, or other markers of chronic diseases. In such cases, a kidney biopsy is necessary to exclude superimposed crescentic glomerulonephritis and to assess the degree of chronic damage.
In acute nephritis, especially in systemic disease-associated nephritis, such as systemic lupus nephritis, microscopic polyangiitis, anti-glomerular basement membrane disease, or polyangiitis granulomatosis, KB is often conducted to study the extent of the kidney damage, which affect therapy plans and treatment. In rare cases, KB can be done in unexplained acute kidney injury [17] despite the high risk of bleeding.
KB is rarely indicated in isolated glomerular hematuria (chronic microscopic hematuria with dysmorphic red blood cells, no proteinuria, normal serum creatinine, and normal blood pressure) since it rarely changes therapy, and the prognosis is good. KB histology in these cases usually shows IgA, Alport syndrome, thin basement membrane changes, or normal kidneys. Unless proteinuria or renal failure are present, KBs are rarely used for definite diagnosis, especially in the USA. [17]
A prospective analysis of 276 native kidney biopsies, including one for hematuria, found that one in thirty-six patients' therapy decisions were entirely affected. [18] For disease progression or proteinuria identification, ongoing monitoring is needed. IgA nephropathy individuals with isolated hematuria may progress with time. [19] Patients with chronic hematuria without evidence of kidney dysfunction features should get a full urological assessment before KB. A KB is advised if a full urologic examination is normal in an individual with isolated microscopic hematuria and for an individual who will be a potential live donor for kidney.
In isolated non-nephrotic proteinuria, KB is not recommended if the individual has low-grade proteinuria (< 500 mg/day) or albuminuria (< 300 mg/day), no glomerular hematuria, normal kidney function, and no clinical or serologic signs of a systemic disease that can cause glomerulonephritis. However, some patients may develop IgA or membrane nephropathy. [20] However, immunosuppressive therapy is not advisable owing to the excellent prognosis of non-nephrotic proteinuria. Nephrologists conduct KB on patients with moderate to high non-nephrotic proteinuria (0.5 to 2 g/day) unless other causes exist, such as persistent diabetes or hereditary kidney disease. If someone is hesitant about KB, in such instances, increased proteinuria, serum creatinine, and abrupt hypertension (HTN) are features to recommend and insist on convincing the patient and family for KB. A total of 249 native kidneys were conducted in Croatia from May 1997 to May 2005, and it found that 95% of the cores successfully retrieved sufficient specimens for histologic diagnosis (11.9 glomeruli). The primary reasons for KB were nephrotic syndrome (NS) (33%), hematuria or non-nephrotic proteinuria (13%), and renal failure (12%). The main glomerulonephritis (GN) was mainly composed of focal segmental glomerulosclerosis (FSGS) in 27%, mesengioproliferative in 13%, IgA nephropathy in 11%, membranous GN (MGN) in 11%, membranoproliferative GN (MPGN) in 5%, crescentic GN in 5%, and minimal change disease (MCD) in 3% of the cores examined. [21]
Kidney transplantation has seen a significant rise worldwide in the past 50 years. However, this rise has come with several challenges, including accurately diagnosing and managing graft dysfunction. Kidney allograft biopsy has been instrumental in addressing this challenge. [22] The allograft KB is a valuable tool for detecting post-kidney transplant acute rejection and guides the management of antibody-mediated or acute cellular rejection. After the necessary therapies are started, a subsequent biopsy is conducted to verify the effectiveness of the therapy in different centers. The allograft period biopsy protocol is the primary method of monitoring in high-risk kidney transplants, such as ABO- or human-leukocyte antigen incompatible transplants, because these patients are at silent immunologic processes that might damage the transplanted kidney. [23]
A study conducted by the United Network for Organ Sharing (UNOS) revealed significant variations in the practices of USA transplant centers concerning the scheduling and execution of surveillance KB of the allograft to detect subclinical rejection. [24] The prevailing time interval for monitoring biopsies was between 3 and 12 months after the transplantation procedure. The graft survival rates at 1 and 3 years were comparable across facilities that conducted biopsies and those that did not. The survey findings revealed the disputes around surveillance KB and the handling of subclinical rejection. [24]
Subclinical rejection is characterized by lymphocyte infiltration in a functioning renal allograft. Rush et al. initially reported the discovery of subclinical rejection during the earliest 3 months post-renal transplantation in the Manitoba Adult Renal Transplant Program. [25] Subclinical rejection was defined as a ≥ 10% elevation in blood creatinine two weeks ahead of the planned KB, along with a histologic Banff score of "ai2at2" or higher, which indicates type 1A acute rejection or more severe rejection. [26] The disagreement surrounding this issue is whether identifying subclinical rejection using a particular biopsy methodology may effectively guide early kidney allograft disease treatment, leading to enhanced long-term graft function and survival. Kidney-transplanted individuals followed for 10 years were identified with subclinical rejection 14 days after transplantation. [27] The findings demonstrated a significant decline in the survival of grafts over 10 years. Consequently, the researchers determined that subclinical rejection could forecast kidney allograft outcomes. A trial was conducted to investigate the advantages of prompt identification of subclinical rejection and administering corticosteroid therapy. [8] The trial studied 72 patients who were divided into two groups for biopsy. The biopsy arm group had KB at 1, 2, 3, 6, and 12 months, while the control group received biopsies only at 6 and 12 months. The biopsy group patients exhibited reduced chronic tubulointerstitial score at 6 months, a decline in acute rejection, and lower plasma creatinine at 24 months compared to the control group. However, if there is suspicion of renal transplant dysfunction, indicated by a rise in blood creatinine or clinical signs (fever, HTN, oliguria, edema, and proteinuria), in that case, it is necessary to perform an allograft biopsy to obtain an accurate histological diagnosis. [28] Several studies have examined the precision of clinical prediction in diagnosing allograft disease based on findings from KB. [29] The findings of those studies indicated that 43% of clinical forecasts were entirely accurate, and 57% had inaccurate predictions. Among these cases, 26% were entirely incorrect, highlighting the importance of KB for precise allograft pathology diagnosis.
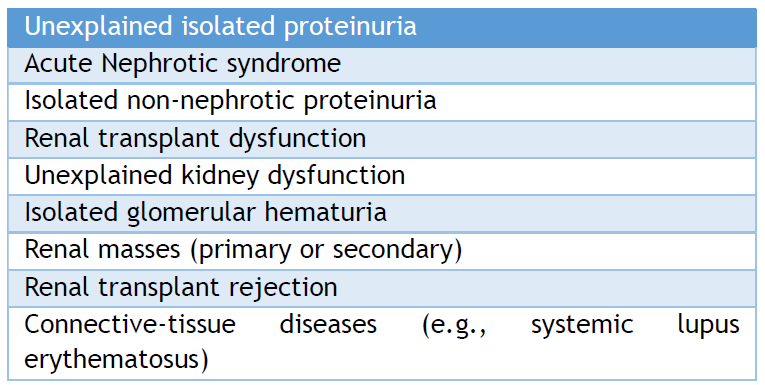
Two retrospective studies were conducted in pediatrics to establish the justifications for KB. The common cause was a glomerular disease that was observed. [30,31] Both studies reported that nephrotic syndrome (32.9%) was the prevalent indication. One study reported that proteinuria (11.4%), asymptomatic hematuria (23.4%), and urine abnormalities in systemic illnesses (15.8%) were the indications for KB. [31] The prevalent causes of glomerular dysfunction were FSGS (20.9%), MPGN (14.6%), immunoglobulin A (IgA) nephropathy (8.9%), MCD (13%), lupus nephritis (LN) (6%), and Henoch-Schönlein nephritis (4%) in a Serbian study. [30] However, another study found that the prevalent findings included FSGS (15%), IgA nephropathy (13.5%), MCD (10%), various stages of LN (8.5%), Henoch-Schönlein nephritis (7.5%), MGN (7.5%), MPGN (6%), postinfectious GN (6%), hemolytic uremic syndrome (5%), tubulointerstitial nephropathies (3.5%), and acute tubular necrosis (2.5%). [31] The main indications for KB are listed in Table 1.
Table 1. Indications of Renal Biopsy
KB is usually a safe procedure once an experienced operator performs it and takes all precautions. However, KB contraindications should be considered carefully before the procedure is conducted.
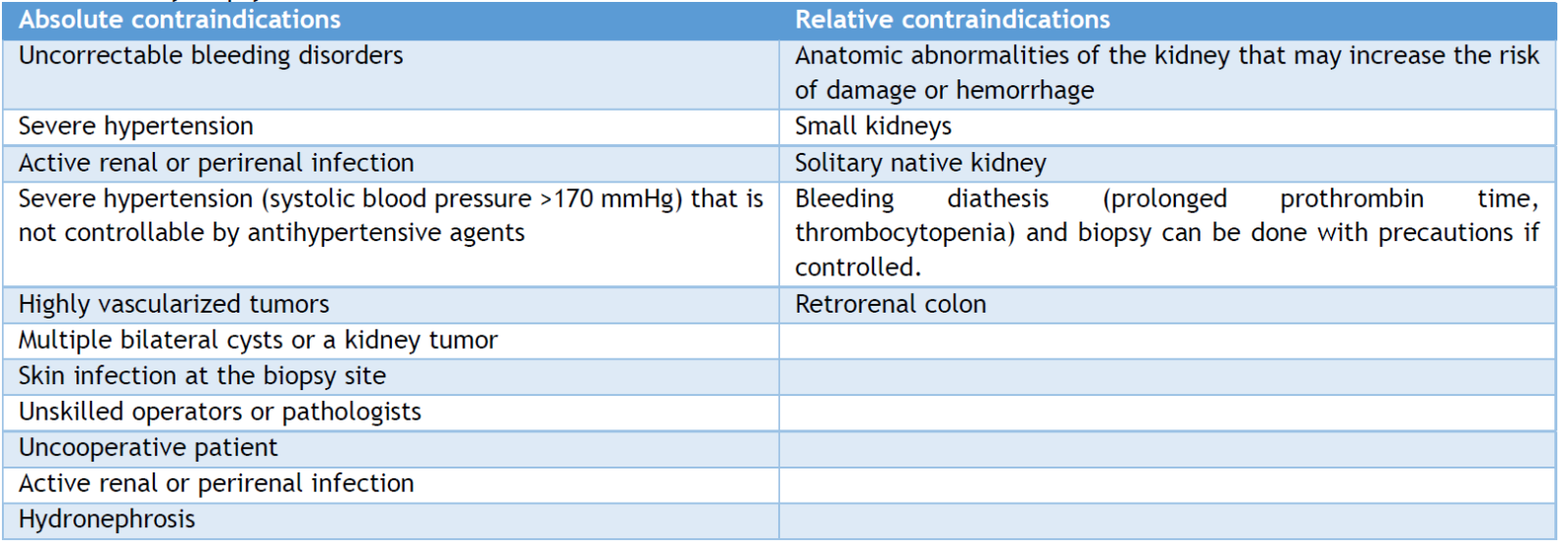
The presence of intravascular coagulopathy, polycystic kidneys, urinary tract obstruction, hydronephrosis, and upper urinary tract infections are absolute contraindications. [32] On the other hand, compromised cardiopulmonary function or unstable hemodynamic status, uncooperative patients, massive obesity, elderly subjects, renal failure, solitary kidney, and severe HTN (> 160/95 mmHg) [32,33] are relative contraindications. All these contraindications and others are reported in Table 2.
Table 2. Kidney biopsy contraindications
Site of kidney biopsy
If no specific areas of interest exist, the biopsy is typically taken from the lower part of the kidney with the patient in the prone position. Typically, the biopsy is executed with ultrasound guidance. [6]
Percutaneous kidney biopsy
PKB is preferred because it is less invasive than alternative techniques and is easy to conduct. A PKB is typically performed under local anesthesia with real-time ultrasonic guidance. However, a computed tomography (CT) scan is an alternative when the kidneys cannot be well visualized [4] or may be preferred based on the operator's experience and radiological equipment availability. Comparing the rates of significant complications between US and CT-guided PKB modalities revealed no significant difference. [34] The effectiveness, methods, and outcomes of CT-directed biopsy have been the subject of research, which has found no statistically significant distinction when compared to US-guided PKB. Currently, most centers utilize automated spring-loaded firearms, and real-time ultrasonography is preferred. [13] Other KB techniques could be considered in complex cases or contraindications to performing PKB. [35]
Open (surgical) kidney biopsy
Open KB may be considered when there is an uncorrectable bleeding diathesis, a solitary kidney, and a lack of an experienced operator or when PKB attempts have failed. It is typically possible to obtain a substantial core or wedge of kidney cortex; the incidence of severe hemorrhage is extremely low, and mortality is uncommon with this method. Other minor complications following surgical KB include fever, atelectasis, and ileus. An open biopsy performed under general anesthesia is linked to a lengthier hospital stay and a larger surgical incision. This method is rarely conducted in stable patients for chronic kidney disease diagnosis.
Laparoscopic Kidney biopsy
For patients who are unable or hesitant to undergo PKB, laparoscopic KB (LKB) is an alternative to open KB. All biopsies were successful in a series of patients who underwent laparoscopy with minimal complications. According to a study evaluating 12 years of LKB experience in China with 104 patients, it was found that retroperitoneal LKB is a secure, dependable, and minimally invasive alternative for patients who cannot undergo PRB. This technique may be increasingly necessary as a helpful addition to PRB in the future. [36] Further confirmatory studies are needed.
Transjugular kidney biopsy
Interventionalists typically perform a transjugular KB (TJKB) in the angiography suite. Although it requires minimal intravenous contrast, it may increase the risk of contrast-induced nephropathy. The main indications of the TJKB method are combined liver or cardiac and KB, morbid obesity, and a single kidney. [37] Theoretically, the risk of perinephric hemorrhage should be less because the kidney capsule is not intentionally pierced. [38] Nonetheless, even with experienced hands, the complication rate of this technique is comparable to that of the percutaneous approach. [39] It was reported that the risk of intrarenal bleeding is high because of arterial trauma due to repeated unintentional capsular perforation. [37,40] Acute kidney injury induced by contrast material has also been reported after this technique [39]. Comparing PKB with TJKB, it was found that the mean number of intact glomeruli per tissue core was 11.2 ± 7.7 and 9.8 ± 7.6 (SD), respectively, when examined through optical microscopy. [41]
The main contraindications for a transjugular KB include bilateral internal jugular vein thrombosis, an allergy to contrast media, and the limited number of experienced operators. [42] Moreover, according to a review published in 2020 on the safety and effectiveness of TJKB, diagnostic tissue was successfully obtained in 90.3% of cases. The review also found that bleeding complications occurred in 22.6% of cases, with 18.2% minor and 4.5% major. Despite these complications, TJKB remains a viable option for obtaining renal tissue for diagnosis, as most complications are self-limiting. [38] This procedure may not be practical for routine kidney biopsies due to the increased cost, need for experienced personnel, and radiologic guidance. However, it may be viable in specific circumstances if the expertise is available.
The selection of a biopsy instrument is primarily a matter of preference. Different biopsy needles are available, including manual and spring-loaded automated needles. Spring-loaded needles with real-time ultrasonic guidance for native and transplant kidney biopsies, due to their superior simplicity of use and increased diagnostic yield, are the favorite. [43] However, the choice is frequently influenced by local resources and availability. It is recommended to use a 16-gauge needle for native kidney biopsies rather than an 18- or 20-gauge needle. An investigation comparing gauge needles for acquiring adequate glomeruli [44] found higher diagnostic yields. The "blind approach" remains valid in the East, whereby an automatic biopsy machine is released without guiding assistance after prior designation on the kidney with US guidance. [44]
The automated and non-automated needles with larger gauges (14 and 16 gauge as opposed to 18 gauge) have yielded more glomeruli per core. [45] It was shown that when an 18-gauge needle is used, the sample size and quality (the number of intact glomeruli) are significantly reduced. A study found that using a 14-gauge needle in PKB of native kidneys resulted in an average of 15.3 glomeruli per core while using an 18-gauge needle resulted in an average of 9.9 glomeruli per core. [46] Nicholson et al. reported that the glomeruli taken by 18-gauge needle compared to 16- or 14-gauge needle were less (9 glomeruli vs. 11 glomeruli vs. 15 glomeruli) and had less diagnostic precision (53 vs. 76 vs 85%) respectively. [47] In addition, others demonstrated that the sample size and the glomeruli number were reduced in native KB samples conducted by 18-gauge needles, with a median of 9 vs12 glomeruli by 16 vs 14-gauge needles, respectively. [48] Another study revealed significantly fewer glomeruli in the core biopsy of 18-gauge needles than 16-gauge needles (12 vs. 19 glomeruli p=0.001). [45] Furthermore, in their report, Peters et al. found that the number of glomeruli was higher in 16-gauge needles compared to 18-gauge needles per biopsy core. In the case of native biopsies, 16-gauge needles showed 11 glomeruli, while 18-gauge needles showed 8 glomeruli per biopsy core (p=0.001). Another report endorsed that the 14- and 16-needle gauge gave similar results as the 18-gauge needle biopsies. [43] Similarly, in biopsies of transplanted kidneys, 16-gauge needles showed 12 glomeruli per core, while 18-gauge needles showed 8 glomeruli per core (p=0.02). [49] Most of the investigators still prefer 14- and 16-gauge needles.
It was reported that the complication rate does not differ between manual and automatic needles of the same gauge. [45] Different studies found no difference in the frequency of complications or the number of glomeruli obtained when using 14-gauge versus 16-gauge automated needles. [50] In addition, a Norwegian registry study of 9288 biopsies revealed no difference in complications between 14- and 16-gauge needles, although 18-gauge needles were associated with a higher incidence of minor complications, primarily significant hematuria. [48] A meta-analysis of 34 retrospective (n = 21) and prospective (n = 13) studies, including 9474 biopsies, revealed a greater need for erythrocyte transfusion in studies employing a 14 gauge compared to either 16 or 18 gauge. [51] Interventional radiologists have performed more PKBs recently, employing smaller 18- and 20-gauge needles. [52] However, using these instrument gauges compromises the diagnostic efficacy of the biopsy without improving patient safety. In a longitudinal study [52] evaluating yield and adequacy, missed diagnoses increased from 2% in 2005 to 14% in 2020.
The number of radiologists who performed KB was only 5% in 2005, which increased to 95% in 2018. Furthermore, it was reported that in smaller-diameter (18- and 20-gauge) biopsy cores in 95% of the procedures, the rate of missed biopsies among nephrologists remained constant over the same period. [52] Given that the glomerular yield in a biopsy sample taken with a 16-gauge is more than those taken with an 18-gauge needle without a significant difference in the risk of complications. The 16-gauge needle is favored for performing a KB in adults. [49,53] Communication between the nephrologist and radiologist regarding the appropriate needle gauge helps to ensure a safe and adequate sampling. [52]
After explaining KB's procedure, indication, and complications, persuade the patient to express questions or worries regarding KB. Let them bring a list of their prescription, over-the-counter medications, and any vitamins or other dietary supplements. Full drug history, especially aspirin, anticoagulants, hepatotoxic medications, and social and family history of any chronic disease such as liver cirrhosis or coagulopathy or diseases in their family must be explored and noted. Patients and families should fully understand and agree to the indication and complications and sign a formal written consent before conducting the KB procedure.
Inform patients that they need to stop taking aspirin temporarily, as well as nonsteroidal anti-inflammatory drugs and anticoagulants, to avoid bleeding complications from the biopsy. [44] Most authors recommend holding antiplatelet and anticoagulants for at least 5-7 days. [54] However, Nayak-Rao suggests that low-risk patients undergoing percutaneous renal biopsies may not need to discontinue antiplatelet agents based on available evidence. [55] The nephrologist should assess the risk case-by-case and balance against the risk of major bleeding; individualized decision-making is important. [55] Mackinnon et al. investigated native PKB complications at facilities that ceased antiplatelet 5 days before PKB (n=75) versus continued (n=60). No biopsies were performed on patients with blood pressure >160/90 mmHg, INR>1.4, or platelet count <100×109/L. Although antiplatelet continuation was linked with an increased absolute hemoglobin drop and patients' percentage with a >1-g/dl drop, elective and urgent PKB patients had similar major complications that required transfusion, radiologic, or surgical intervention. [54] In a 2002–2008 single-center study of 5832 allograft and native PKBs, Atwell et al. observed no distinction in hemorrhage risk between aspirin and no aspirin stoppage within 10 days after biopsy. [56] In a meta-analysis report, patients who received antiplatelet medications for ≥7 days had the same transfusion rate (n=2116 biopsies) as those who did not (seven trials; n=4009). [51] Given the limited evidence and the fact that most kidney biopsies are elective, it is advisable to hold antiplatelet drugs for 7 days before the surgery when feasible. [2] Patients on chronic use of warfarin or low molecular weight heparin have logistical challenges but may safely undertake a PKB with a short duration of anticoagulation or a heparin bridging.
The only visible evidence-based found for antithrombotic treatment recommendations for perioperative care is reported by Douketis et al.. [57] No evidence exists on how newer anticoagulants (Direct oral anticoagulants) affect PRB bleeding complications. Laboratory tests can identify potential coagulopathies. Bleeding time can be used to evaluate the time of platelet aggregation; if there is advanced renal failure or prolonged bleeding time, administering desmopressin acetate, cryoprecipitate, and estrogen was shown to minimize the risk of bleeding. [58] According to one of the authors' experiences, Assessing bleeding and prothrombin time is a good practice to minimize the bleeding risk and rate.
After conducting the KB, an immediate light microscopy examination of the KB specimen for adequacy and the number of glomeruli obtained is usually a widespread practice by nephrologists. Further evaluations by light and electron microscopy examinations, immunofluorescence, and immunoperoxidase investigations should be conducted. Immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA), C3, C4, C1q, kappa chain, lambda chain, albumin, and fibrin all must be assessed. By doing these investigations and blood tests, most of the time, diagnosis is established. In cases where they are available, special investigations such as evaluation of serum amyloid A deposits, IgG subclasses (IgG1 to 4), phospholipase A2 receptor (PLA2R), collagen chains (alpha 3, 4, and 5), heat shock protein, and mass spectrometry may be helpful. If glomeruli were not present in the original sample, immunofluorescence microscopy on paraffin sections metabolized with pronase might salvage a diagnosis. [59]
Studies have shown that in approximately 50% of instances, the electron microscopy examination gave useful diagnostic information beyond that gained through light microscopy and provided the main justification for the widespread deployment of electron microscopy. [60] Recent studies have shown that electron microscopy has yielded comparable outcomes. [61]
Diagnosing conditions such as postinfectious glomerulonephritis, thin basement membrane nephropathy, membranous nephropathy, Alport syndrome, HIV-associated nephropathy, amyloidosis, immunoglobulin deposition diseases, fibrillary glomerulonephritis, and immunotactoid glomerulopathy requires an electron microscopy and immunofluorescence examination.
The abdominal US is used to assess any significant change in the biopsied kidney size and any evidence of bleeding. The abdominal US can be conducted after the biopsy and before discharge; however, it is not routine. In case of severe hematuria, pain, or renal dysfunction, urgent abdominal US must be done promptly to exclude hematoma, bleeding, or organ perforation. Inform the patient that they must lie on the back with a supporting cloth underlying the biopsy site. [62] After the procedure, patients may have to stay overnight in the hospital. They should spend at least 2 hours post-biopsy to observe pulse rate, respiratory rate, and blood pressure. Besides intravenous fluid, some physicians give diuretics to assess for passing urine and assessing the presence and severity of hematuria. Patients will be given pain medication other than nonsteroidal anti-inflammatory drugs. During their hospital stay for observation, blood workup, vital signs, and urine should all be monitored. [62] Patients are advised to refrain from intense activity, such as heavy object lifting, for 2 weeks after KB. [62]
By utilizing real-time ultrasound alongside an automated 16-gauge automatic biopsy machine and adhering to specific safety measures, a skilled operator can successfully perform PKB with no or minimal complications. It is recommended that PKB safety be evaluated and any late complications be anticipated through observation two hours after the procedure, ensuring a safer and more cost-effective outcome was reported in a study. [63] A study has determined that the most favorable time to assess the safety of patients and anticipate any potential complications is by observing patients post-PKBs for 2 hours. This approach not only guarantees the safety of patients but also proves to be more cost-effective. [64]
The observation period's duration after PKB must be clearly defined because various observation times were reported. It was reported that 24-hour observation is recommended to detect any complication, [65,66] although others did not recommend that. [67] However, another study concluded that hospitalization was unnecessary. [68] Minor complications such as discomfort are more likely to occur in younger, healthy patients who experience hematuria before the biopsy. Japanese 2020 kidney biopsy guidelines recommend bed rest is needed for 6–24 hours. Eating and drinking must be conducted while the patient is lying down after KB, as well as bed urination and defecation. Urinary bladder catheterization for problematic urination is advisable. After KB, fever may arise, which may indicate hematoma development. [66]
Post-KB, the patient needs to rest in a supine position for at least 6-8 hours. The vitals, urine output, and color should be checked to evaluate the presence of gross hematuria. If there are no signs of bleeding within the first 6 hours, the patient may sit up because most complications occur within 6-8 hours. [32] Approximately 20% of complications occur during the first 8 hours post-KB, according to a study involving 394 patients [69]. Whittier and Korbet reported that in 750 KB, < 8 hours of post-KB observation was suboptimal, potentially failing to detect as many as 33% of complications. Forty-five out of 750 cases (6.6%) had a significant complication at and after 8 hours post-KB. [70] Thirty were diagnosed after 8 hours of post-KB observation. The remaining fifteen were identified between 9 and 14 hours of post-KB observation time. Other four studies have presented divergent findings. A cohort study of 118 patients documented only minimal complications. [71] A Nigerian study documented the absence of complications in a cohort of 20 patients, [72] supported by the same result by Murphy BF et al. [73] A total of 178 outpatient KBs were examined, and only 13.2% experienced minimal complications; these included 4 patients who presented with extensive hematuria, 16 patients who presented with small perinephric blood collection, and 3 patients who presented with hematoma and hematuria, [74] which did not require interventions. It has been reported that an outpatient, real-time, ultrasound-guided PKB is a safe and effective way to minimize the need for post-KB hospitalization. The procedure can result in significant cost savings without any increased risk of complications. [68] Patient selection is crucial, as KB of patients had high serum creatinine levels, and females with acute kidney injury had higher complication rates. [51] Future studies should focus on identifying risk factors to improve safety and determine the optimal safe post-KB observational time, whether doing Doppler US post-KB when to do it, and whether it is a proper practice or not.
Post-KB, infection is very unlikely; however, It is imperative to address discomfort and bleeding as they are critical concerns that demand urgent attention and must not be underestimated, and the patient must consult their physician if any changes or symptoms or signs appear after the KB. [75] The KB complications risk and rate remain limited due to the heterogeneity, reporting bias, and variations in complications definitions in various studies over the last 50 years.
In a systematic review that included 118,604 native PKBs [76], the bleeding incidence complications were perinephric hematomas (11%), biopsy site pain (4.3%), transient macroscopic hematuria (3.5%), erythrocyte transfusion was required in (1.6%), only 0.3% of cases needed surgical interventions. Death occurred in 0.06%, and nephrectomy was required for only 0.01%. [51] A study investigating 249 PKB complications found that the most common complications reported were perirenal hematoma (without clinical symptoms) in 3.6% of cases and macrohematuria in 1.2%. No bleeding complications that required therapeutic radiologic intervention or blood transfusion were reported. Surgical intervention was necessary in just one patient, accounting for 0.4% of the cases. [21]
In the lumbar and renal areas, discomfort and numbness occur when the anesthesia action ends after the KB. The pain may require painkillers. However, acute discomfort may suggest a significant problem requiring more diagnostic investigations. Post-KB's most frequent side effect is asymptomatic, microscopic hematuria that disappears spontaneously over a few days. [77] In 3% of KBs, gross hematuria resolved within hours; however, it persisted for days in a few patients. Gross hematuria may induce a reduction in hemoglobin concentration, necessitating a blood transfusion, or lead to clot development with or without obstructive uropathy and renal failure. In one study, acute anemia occurs in > 50% of patients post-KB, manifesting in a hemoglobin concentration drop of ≥ 1 g/dL. [78] In comparison, others reported a 10% drop in hemoglobin of ≥ 2 g/dL, increasing the risk of complications. [79,80] However, it rarely requires red blood cell transfusion. [81]
Perinephric hematoma is frequently asymptomatic. It is usually found on a renal ultrasound following a biopsy and does not lead to significant problems. Prospective studies found perinephric hematoma in 90% of patients 24-72 hours and 15% immediately after the KB. Most perinephric hematomas are tiny, asymptomatic, and heal spontaneously in a few months. Approximately 2% of cases result in clinically significant consequences, such as severe lower back pain, a decrease in hemoglobin levels, or bleeding that necessitates a blood transfusion. The lack of hematoma at one hour post-KB strongly suggested a straightforward course. [82,83]
A report by Waldo et al. found that 95% of individuals with no perinephric hematoma one hour after KB had not developed severe problems; however, some patients with hematoma developed serious problems later. [84] Hence, it was advised by this study to conduct kidney US during the first hour after PKB [84]. Another study reported that post-KB peri-nephric hematoma was present in 86% of cases, and they were small-sized hematomas (<2 cm). In the same study, about 13% of cases had > 2 cm-sized hematomas [85] detected by post-KB US. Massive bleeding around the kidney or at the retroperitoneal space can cause hypotension and even shock and may require a blood transfusion. As blood continues to collect in the retroperitoneal space, surgical intervention may be needed to relieve compression and stop bleeding; however, it is rarely required. [86] Large perinephric hematoma might cause compression and ischemia of the kidney (Page kidney), impairing kidney function and survival in severe cases.
Reviewing 34 studies that included 9,474 KBs, emphasizing post-KB bleeding. [76] Every KB examined in this study was performed utilizing automated biopsy instruments guided by CT or US imaging. Protocol biopsies of the kidney were performed by the Kidney Precision Medicine Project (KPMP) [76] for research objectives. The relevant publications published by the KPMP group from 2011 to 2017 were incorporated into the prior meta-analysis. The most recent systematic review, which examined Published articles on renal biopsies from January 1983 to March 2018, reviewed and revealed that bleeding post-PKB was rare. [76] However, the massive bleeding after PKB might be critical, leading to graft thrombosis, acute failure, and HTN due to renin-angiotensin-aldosterone system activation. [87,88] Previous studies have suggested that the incidence of severe bleeding was similar after PKB, irrespective of the facility setting (outpatient or inpatient). [89]
Arteriovenous fistula (AVF) is a rare known complication of KB that usually resolves spontaneously. [90] Persistent hematuria after three days mostly indicates an AVF development. [91] This complication may occur in almost 18% of KB patients. [92] In most cases, however, the AVF is symptomless and resolves spontaneously. AVF patients may develop symptoms like hematuria, kidney insufficiency, and HTN. Proper management is crucial to prevent complications. Selective angioembolization is indicated to stop bleeding in severe cases. [93] AVF may sometimes create aneurysms, which can cause high blood pressure, heart failure, and renal failure. Consistent gross hematuria, abdominal bruit, and palpable thrill indicate this complication. [94]
AVF rate in transplanted and native kidneys demonstrated a higher incidence than previously reported. [95,96] Needle size is important because the larger the needle, the higher the risk of AVF formation. [95] Most lesions did not require intervention, and 95.4% resolved spontaneously within the following three months. No specific symptoms, such as gross hematuria, hemodynamic changes owing to high shunt flow, or significant kidney dysfunction, were observed in most cases. In cases of AVF, a Doppler ultrasound and CT-angiography should be performed to assess and follow up. [97] Treating severe symptoms involves super selective transcatheter arterial embolization or, rarely, surgery. [98]
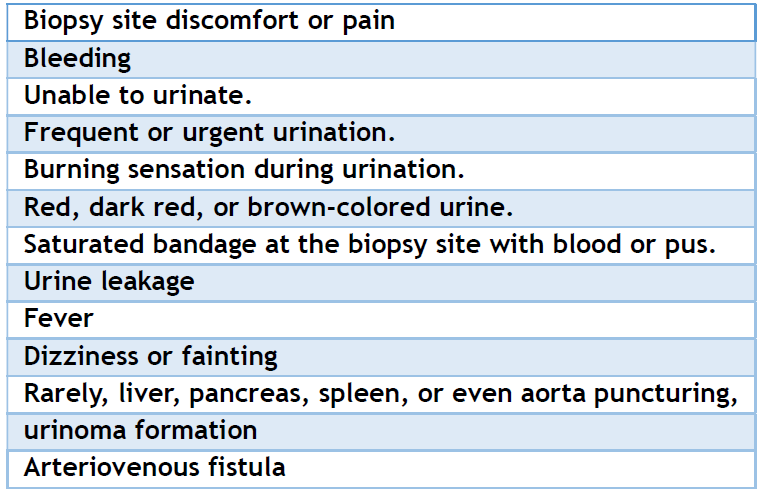
It has been claimed that repeated renal biopsy may cause fibrosis at the side of the tissue biopsied. Considerable damage was not reported; however, repeated multiple biopsies are not advisable, especially in a single native kidney or transplanted kidney, and if one good functioning kidney and the other kidney function is compromised. Multiple kidney fibrotic sites might increase blood pressure and cause HTN. [99] Rarely, the damage caused by the biopsy needle to the liver, spleen, aorta, and urinary system puncture causes severe complications; however, minor bleeding and urinoma may occur. [100] The intra-renal pseudoaneurysm was reported post-percutaneous KB in lupus patients, which may present with acute pain or hematuria. [101] A summary of KB complications is presented in Table 3.
Table 3. Expected complications.
A study revealed concerns about conducting surveillance biopsies and managing subclinical rejection. [24] Subclinical rejection diagnosis in the first three months following kidney transplantation was initially reported by the Manitoba Adult Renal Transplant Program. [25] The lymphocyte's existence in a normally functioning transplanted kidney is the defining characteristic of subclinical rejection. The timing and execution of transplanted KBs for detecting subclinical graft rejection varied widely across the USA transplant hospitals, according to a survey by the United Network for Organ Sharing (UNOS). [102] Surveillance biopsies were most frequently performed between three and twelve months after transplant. The one and 3-year graft survival was comparable between centers that performed biopsies and those that did not.
In a study, individuals with subclinical rejection identified 14 days after transplantation were followed up for 10 years. [27] According to conclusions drawn from this study, the researchers found that subclinical rejection can predict transplant outcomes. The study's findings suggest that subclinical rejection is crucial in determining transplant success and graft survival, evidenced by a significant decline in graft survival during the study interval. [27] Another study reported that the prevalence of subclinical rejection, according to the Banff criteria, is about 30% had acute kidney rejection attack in the first 3 months. [103] Furthermore, the same study reported the advantages of spotting subclinical rejection early and administering corticosteroids. [103]
Results of this study showed that those who underwent biopsies had lower incidences of acute rejection, chronic tubulointerstitial scores, and serum creatinine levels. If there are signs of renal transplant failure, such as increased plasma creatinine or clinical symptoms including fever, edema, HTN, oliguria, and proteinuria, obtaining a biopsy of the allograph is crucial for an accurate diagnosis [28]. It was reported that clinical predictions regarding allograft pathology diagnosis following KB are inaccurate 57% of the time, with only 43% being completely accurate. Therefore, utilizing a KB to diagnose allograft diseases accurately is imperative since 26% of the inaccurate predictions were completely wrong [29]. The identification of acute transplanted allograft rejection and the treatment plan for acute cellular or antibody-mediated rejection are both helped by allograft KB. A repeat biopsy helps ensure a satisfactory response to treatment after the proper treatments have been started. The schedule of allograft interval biopsies is crucial for the surveillance of high-risk transplant patients, particularly those with ABO- or HLA-incompatible recipients involved in kidney transplants. Such patients may experience impaired graft function caused by concealed immunologic processes [23]. Protocol allograft KB is controversial; however, it is indicated in the case of clinical or biochemical rejection. Further research is necessary to assess this controversy.
CKD affects up to 10-15% of the world population, [104,105] affecting 697,5 million with different CKD stages, with almost 1.2 million CKD-related deaths. [106] KB in CKD is not a standard procedure. However, in some cases, such as new onset hematuria, proteinuria, an increase in proteinuria, and rapid deterioration of stable CKD, KB is needed for prognosis, detecting the underlying pathology, and planning therapy. [107]
Recent research found that after a median follow-up time of 37.6 months, the biopsy group's estimated GFR raised by > 10 ml/min/1.73 m². Interestingly, the non-biopsy group declined by about the same value. Patients with KB had a greater survival rate (P < 0.001). Over a day of urine protein excretion > 1 g/day, the study predicted that there would be reduced renal outcomes in the group that did not undergo biopsy. However, no significant reduction was predicted in the group that did undergo KB [107]. This could be due to the detection of the underlying cause and the promotion of therapy to the cause.
The gold standard for kidney disease diagnosis is KB. However, KB is not commonly recommended for every impaired renal function because reduced renal function may increase bleeding risk, and the biopsy core may not provide adequate information for diagnosing and therapy modification in CKD. [108] However, neglecting renal biopsy in renal impairment patients may miss curable interstitial nephritis, [109] which may swiftly advance to ESRD without therapy. KB is essential for disease progress and severity assessment. Thus, understanding renal impairment patients' baseline and pathological features is crucial, even in CKD patients.
Most nephrologists now empirically determine if KB is indicated for CKDs with compromised renal function based on proteinuria, hematuria, etc. To our knowledge, no pathological investigations have examined chronically impaired renal function patients and their kidney outcomes by KB. Although KB is the gold standard for kidney disease diagnosis, it has not been studied thoroughly in CKD patients. A recent study concluded that CKDs with rapidly decreasing renal function should have KB for pathological diagnosis, particularly those with daily urine protein excretion > 1 g/day. [107] Despite the rarity of studies investigating the benefit of KB in CKDs, we think that KB may still have a role in CKD underlying cause diagnosis and prediction of CKD progression rate. Hence, further studies are required to investigate this thought.
It has been stated that outpatient PKB is a safe option for low-risk patients compared to inpatient PKB, and the cost is lower. [110] Communities with high rates of renal disease are expected to have a proportional amount of renal biopsy requests. Despite the growing reports of kidney disease, the decline in kidney biopsies conducted in African countries is a concern. [111,112] A study has found that the decline in requests for renal biopsies may be due to a shortage of professionally trained staff, inadequate health insurance schemes, high costs, and insufficient facilities. Given the significance of kidney biopsies, it is crucial to make concerted efforts to install this important investigative procedure in local tertiary hospitals. [113] The cost of a biopsy varies depending on whether it is done on an outpatient or inpatient basis.
The median cost for an outpatient PKB is 1,968 USD, while an inpatient KB costs 3,178 USD. A study found that performing the biopsy as an outpatient procedure for pediatric patients is just as safe as an inpatient procedure, and it can save over 1,000 USD per biopsy when they were observed only for 2 hours at the Cleveland Clinic Children's Hospital. [114] In the USA, PKB costs range from 1.824 to 4.340 USD. In India, PKB costs range from around 5.000 to 20.000 Rs (60.91 to 243.62 USD). Another Indian study reported that the total cost of PKB is 40,000 Rs (487.49 USD) [6]. The average price of PKB in Deutschland facilities is 19229 Euro (3195-88750 USD), while PKB costs between 200-300 British Pounds in the UK. In China, the average cost of PKB is between 3000-7806 USD, with a maximum price of 6000 USD. The cost of PKB in Nigeria ranges from 190-2.022 USD. In Gulf countries, for example, in the United Arab Emirates, it costs between 165-330 USD. PKB may be unavailable in some places due to a scarcity of expert practitioners or pathologists or a lack of electron microscopy and immunological reagents and studies. Interestingly, a study conducted between May 2012 and September 2015 compared the cost of kidney biopsies using KB methods in 78 patients. The study revealed that the cost of ultrasound (US)-guided hospital-based (UGHB), CT-guided hospital-based (CTG), and US-guided office-based (UGOB) PKB for renal masses was 4,598, 4,470, and 2,129 USD, respectively. This includes facility, professional, and pathology fees. [115] The same study concluded that UGOB and PKB have equivalent diagnostic and complication rates and are more cost-effective than UGHB or CTG renal biopsy.
Although KB is necessary and safe, many patients are still reluctant to undergo the procedure. This is often due to poor communication with their healthcare provider, anxiety about the procedure, high costs, and limited availability of expert operators and histopathologists. Furthermore, in some cases, patients and families agree with KB, but due to a lack of necessary equipment, pathologists, electron microscopy, immune histology, and US or CT machines hinder the procedure. Additionally, in many situations, the potential for serious complications from KB can also be a concern for patients, affecting the conductance rate of KB. Accordingly, international communities need to support these areas that lack the necessary facilities and expertise to conduct KB. This will help to ensure that patients receive the diagnostic benefits of KB, which can be vital for their health and well-being.
A crucial investigative tool in the context of kidney disease diagnosis is KB. Various methodologies exist for KB, such as PKD, TJKB, open (surgical) KB, and LKB. As stated in the preceding sections, every method has indications and potential complications. PKB is a prevalent technique due to its ability to be performed on an outpatient basis, its cost-effectiveness compared to alternative methods, and the absence of substantial complication variation.
Before conducting KB, the health provider team must notify the patient and family and fully explain the procedure's indication and possible complications. In simple words, seniors must fully explain this information to the family and patients. The biopsy should only be done to guide therapy, diagnose and adjust treatment, or predict prognosis. Before biopsy, patients must be thoroughly prepared and any major contraindications eliminated. Abdominal US is necessary before a KB to ensure kidney visibility and location, exclude anatomical anomalies, and assess the risk-benefit ratio. [1] Small kidneys and poor corticomedullary differentiation reflect ambiguous chronicity, restricted renal disease reversibility, and low biopsied tissue piece histopathological yield, preventing KB from being conducted. Additionally, differentiating the kidneys from surrounding retroperitoneal tissues may be challenging.
Some patients may worry about kidney tissue removal affecting renal function. Fortunately, one research calculated that biopsy-induced GFR reduction in stable transplant patients is only 0.77 mL/min. [116] However, In native kidney biopsy, a study reported that the estimated GFR improved significantly after KB compared with the non-biopsied group (p < 0.001) during 5 years of follow-up. [107]
While KB is crucial for the diagnosis of numerous intrarenal diseases, it also aids in the identification of systemic diseases that involve the kidneys, such as lupus nephritis that might occur with systemic lupus nephritis [117,118] or myeloma kidney in multiple myeloma [14]. Moreover, KB is indispensable for the diagnosis and prognosis of interstitial kidney disease progression [119]. Kidney transplantation is associated with both acute and chronic rejection [120]. In addition to clinical and laboratory indicators of rejection, KB is crucial for determining the nature of rejection and the allograft's outcome [121]. Moreover, positive KB of allograft rejection impacts the rejection treatment strategy. The practice of performing KB post-kidney transplantation is a subject of controversy; however, it should not be neglected. This matter was comprehensively addressed earlier in this article.
CKD is a prevalent disease on a global scale. CKD is frequently induced by diabetes and HTN; nevertheless, it is crucial to also consider interstitial kidney diseases and systemic illnesses as potential contributors. When renal function declines abruptly, and for no apparent reason, KB is indicated in such situations. Nevertheless, it is crucial to thoroughly evaluate the corticomedullary differentiation and kidney size to ascertain the sufficiency of the biopsy core and the incidence of complications. KB in CKD is still controversial among nephrologists; however, in the situations mentioned, KB is indicated, but extreme cautions must be implemented.
The KB cost is a critical and trending topic. PKB is the most economical and secure method of KB. However, it remains prohibitively expensive and unavailable in specific communities. It can cost between 200 and 5,000 USD worldwide. This cost might not be high in developed nations and those with good health insurance systems or free health services. Safe KB requires, in addition to cost, accessibility in impoverished communities, pathology expertise, immunological studies, immunofluorescence studies, and US and CT equipment.
Supporting communities that do not practice KB by providing essential equipment and training for local personnel is crucial. We believe that the World Health Organization and health authorities of the developed nations can significantly assist developing and underdeveloped nations in improving their health by providing free health services, excellent health insurance schemes, CKD prevention, and transplant patient care programs. Other forms of assistance could be dispatching pathologists, expert nurses, physicians, and equipment to low-income nations. In addition, providing scholarships for the training of local employees is an essential form of assistance.
Limitation
One limitation is that we restricted our search to only three engines (Google, Google Scholar, and PubMed), which may have resulted in fewer evidence sources. However, the current review is already lengthy; incorporating results from other search engines could potentially lengthen it and make it more challenging to follow.
An ultrasound or CT-guided PKB is safe and informative, but it has rare complications. Pain, discomfort, and minor bleeding are the most common, although they are usually self-limiting. If a damaged vessel causes bleeding, embolization is an effective treatment. Needle sizes 14 and 16 have reduced complication rates and significantly increased the likelihood of obtaining the necessary glomeruli compared to other needle sizes. However, pericapsular hematomas can occur, which may require drainage. Although rare, continuous bleeding may require a blood transfusion, embolization, and surgical intervention. However, kidney loss and death post-PKB have been reported only in a small percentage. Improving the governmental facilities' delivery of services and training local health providers are crucial to increasing the number of the indicated KB, making KB available at a lower cost, reducing complications and risks, and preventing chronic kidney diseases and their progression rate.
ACKNOWLEDGMENT
We acknowledge the support from the Open Libyan University.
AUTHORS’ CONTRIBUTION
All authors have significantly contributed to the work, whether by conducting literature searches, drafting, revising, or critically reviewing the article. They have given their final approval of the version to be published, have agreed with the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
References
- Hull KL, Adenwalla SF, Topham P, Graham-Brown MP. Indications and considerations for kidney biopsy: an overview of clinical considerations for the non-specialist. Clin Med (Lond). 2022;22(1):34-40.
- Hogan JJ, Mocanu M, Berns JS. The Native Kidney Biopsy: Update and Evidence for Best Practice. Clin J Am Soc Nephrol. 2016;11(2):354-62.
- Mattiazzi AD, Cortesi CA, Patil RJ, Carias Martinez KG, Sedki M, Cabeza Rivera FH, et al. Percutaneous Ultrasound-Guided Kidney Transplant Biopsy Outcomes: From the Nephrologist to the Radiologist Standpoint. Kidney360. 2022;3(10):1746-53.
- Granata A, Distefano G, Pesce F, Battaglia Y, Suavo Bulzis P, Venturini M, et al. Performing an Ultrasound-Guided Percutaneous Needle Kidney Biopsy: An Up-To-Date Procedural Review. Diagnostics (Basel). 2021;11(12):2186.
- Iversen P, Brun C. Aspiration biopsy of the kidney. Am J Med. 1951;11(3):324-30.
- Rathod KR, Popat BA, Pandey A, Jamale TE, Hase NK, Deshmukh HL. Safety and effectiveness of transjugular renal biopsy: A single center study. Indian J Nephrol. 2017;27(2):118-23.
- Redfield RR, McCune KR, Rao A, Sadowski E, Hanson M, Kolterman AJ, et al. Nature, timing, and severity of complications from ultrasound-guided percutaneous renal transplant biopsy. Transpl Int. 2016;29(2):167-72.
- Liang D, Zhang H, Yang M, Ji H, Chen G, Yu N, et al. Massive hemorrhage after percutaneous kidney biopsy caused by renal artery malformation: a case report and literature review. BMC Surg. 2020;20(1):256.
- Lees JS, McQuarrie EP, Mordi N, Geddes CC, Fox JG, Mackinnon B. Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin Kidney J. 2017;10(4):573-577.
- MacGinley R, Champion De Crespigny PJ, Gutman T, Lopez-Vargas P, Manera K, Menahem S, Saunders J, See E, Voss D, Wong J. KHA-CARI Guideline recommendations for renal biopsy. Nephrology (Carlton). 2019;24(12):1205-1213.
- Dhaun N, Bellamy CO, Cattran DC, et al. Utility of renal biopsy in the clinical management of renal disease. Kidney Int. 2014;85(5):1039–48.
- Ball RP. Needle (Aspiration) Biopsy. JAMA. 1936;107(17):1381.
- Mukhtar KN, Mahmood SN, Umair SF. CT guided percutaneous renal biopsy versus ultrasound guided for obtaining adequate tissue. J Pak Med Assoc. 2012;62(9):880-2.
- Habas E, Akbar R, Farfar K, Arrayes N, Habas A, Rayani A, et al. Malignancy diseases and kidneys: A nephrologist prospect and updated review. Medicine (Baltimore). 2023;102(15):e33505.
- Rosner MH, Jhaveri KD, McMahon BA, Perazella MA. Onconephrology: The intersections between the kidney and cancer. CA Cancer J Clin. 2021;71(1):47-77.
- Bolufer M, García-Carro C, Blasco M, Quintana LF, Shabaka A, Rabasco C, et al. Kidney Biopsy in Patients with Cancer along the Last Decade: A Multicenter Study. J Clin Med. 2022;11(10):2915.
- Fuiano G, Mazza G, Comi N, Caglioti A, De Nicola L, Iodice C, et al. Current indications for renal biopsy: a questionnaire-based survey. Am J Kidney Dis. 2000;35(3):448-57.
- Richards NT, Darby S, Howie AJ, Adu D, Michael J. Knowledge of renal histology alters patient management in over 40% of cases. Nephrol Dial Transplant. 1994;9(9):1255-9.
- Szeto CC, Lai FM, To KF, Wong TY, Chow KM, Choi PC, et al. The natural history of immunoglobulin a nephropathy among patients with hematuria and minimal proteinuria. Am J Med. 2001;110(6):434-7.
- Hall CL, Bradley R, Kerr A, Attoti R, Peat D. Clinical value of renal biopsy in patients with asymptomatic microscopic hematuria with and without low-grade proteinuria. Clin Nephrol. 2004;62(4):267-72.
- Horvatić I, Hrkać A, Zivko M, Kozjak D, Galesić K. Value of ultrasound-guided percutaneous renal biopsy in diagnosis of the renal diseases]. Acta Med Croatica. 2007;61(4):399-403.
- Mubarak M, Raza A, Rashid R, Shakeel S. Evolution of human kidney allograft pathology diagnostics through 30 years of the Banff classification process. World J Transplant. 2023;13(5):221-238.
- Dörje C, Mjøen G, Strøm EH, Holdaas H, Jenssen T, Øyen O, et al. One-year protocol biopsies from ABO-incompatible renal allografts compared with a matched cohort of ABO-compatible allografts. Clin Transplant. 2015;29(3):268-76.
- Mehta R, Cherikh W, Sood P, Hariharan S. Kidney allograft surveillance biopsy practices across US transplant centers: A UNOS survey. Clin Transplant. 2017;31(5).
- Rush DN, Henry SF, Jeffery JR, Schroeder TJ, Gough J. Histological findings in early routine biopsies of stable renal allograft recipients. Transplantation. 1994;57(2):208-11.
- Rush D. Protocol transplant biopsies: an underutilized tool in kidney transplantation. Clin J Am Soc Nephrol. 2006;1(1):138-43.
- Choi BS, Shin MJ, Shin SJ, Kim YS, Choi YJ, Kim YS, et al. Clinical significance of an early protocol biopsy in living-donor renal transplantation: ten-year experience at a single center. Am J Transplant. 2005 Jun;5(6):1354-60.
- Ahmad I. Biopsy of the transplanted kidney. Semin Intervent Radiol. 2004;21(4):275-81.
- Al-Awwa IA, Hariharan S, First MR. Importance of allograft biopsy in renal transplant recipients: correlation between clinical and histological diagnosis. Am J Kidney Dis. 1998;31(6 Suppl 1):S15-8.
- Paripović D, Kostić M, Kruščić D, Spasojević B, Lomić G, Marković-Lipkovski J, et al. Indications and results of renal biopsy in children: a 10-year review from a single center in Serbia. J Nephrol. 2012;25(6):1054-9.
- Printza N, Bosdou J, Pantzaki A, Badouraki M, Kollios K, Ghogha Ch, et al. Percutaneous ultrasound-guided renal biopsy in children: a single centre experience. Hippokratia. 2011;15(3):258-61.
- Visconti L, Cernaro V, Ricciardi CA, Lacava V, Pellicanò V, Lacquaniti A, et al. Renal biopsy: Still a landmark for the nephrologist. World J Nephrol. 2016;5(4):321-7. doi: 10.5527/wjn.v5.i4.321.
- Eiro M, Katoh T, Watanabe T. Risk factors for bleeding complications in percutaneous renal biopsy. Clin Exp Nephrol. 2005;9(1):40-5.
- Vu T, Shin B, Mittal A, Sarwani N, McGillen KL. Ultrasound Versus Computed Tomography-Guided Native Parenchymal Kidney Biopsies for Hospitalized Patients: Comparison of Clinical Outcomes and Complications. Ultrasound Q. 2022 Dec 1;38(4):328-33.
- Bandari J, Fuller TW, Turner Іі RM, D’Agostino LA. Renal biopsy for medical renal disease: indications and contraindications. Can J Urol. 2016;23(1):8121-6.
- Zou G, Chen H, Zhou X, Li W, Zhuo L. Retroperitoneal laparoscopic renal biopsy: an 8 year experience at a single centre. Int Urol Nephrol. 2023 Apr;55(4):969-973.
- Meyrier A. Transjugular renal biopsy. Update on hepato-renal needlework. Nephrol Dial Transplant. 2005 Jul;20(7):1299-302.
- St Jeor JD, Reisenauer CJ, Andrews JC, Fleming CJ, Misra S, Takahashi EA. Transjugular Renal Biopsy Bleeding Risk and Diagnostic Yield: A Systematic Review. J Vasc Interv Radiol. 2020;31(12):2106-2112.
- Misra S, Gyamlani G, Swaminathan S, Buehrig CK, Bjarnason H, McKusick MA, Andrews JC, Johnson CM, Fervenza FC, Leung N. Safety and diagnostic yield of transjugular renal biopsy. J Vasc Interv Radiol. 2008;19(4):546-51.
- Thompson BC, Kingdon E, Johnston M, Tibballs J, Watkinson A, Jarmulowicz M, Burns A, Sweny P, Wheeler DC. Transjugular kidney biopsy. Am J Kidney Dis. 2004 Apr;43(4):651-62.
- Cluzel P, Martinez F, Bellin MF, Michalik Y, Beaufils H, Jouanneau C, et al. Transjugular versus percutaneous renal biopsy for the diagnosis of parenchymal disease: comparison of sampling effectiveness and complications. Radiology. 2000;215(3):689-93.
- Sam R, Ing TS. Transjugular renal biopsy: when to do it and when not to? Int J Artif Organs. 2001;24(9):595-7.
- Korbet SM. Percutaneous renal biopsy. Semin Nephrol. 2002;22(3):254-67.
- Alwall N. Aspiration biopsy of the kidney, including i.a. a report of a case of amyloidosis diagnosed through aspiration biopsy of the kidney in 1944 and investigated at an autopsy in 1950. Acta Med Scand. 1952;143(6):430-5.
- Mai J, Yong J, Dixson H, Makris A, Aravindan A, Suranyi MG, Wong J. Is bigger better? A retrospective analysis of native renal biopsies with 16 Gauge versus 18 Gauge automatic needles. Nephrology (Carlton). 2013;18(7):525-30.
- Kim D, Kim H, Shin G, Ku S, Ma K, Shin S, Gi H, Lee E, Yim H. A randomized, prospective, comparative study of manual and automated renal biopsies. Am J Kidney Dis. 1998;32(3):426-31.
- Nicholson ML, Wheatley TJ, Doughman TM, White SA, Morgan JD, Veitch PS, et al. A prospective randomized trial of three different sizes of core-cutting needle for renal transplant biopsy. Kidney Int. 2000;58(1):390-5.
- Tøndel C, Vikse BE, Bostad L, Svarstad E. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol. 2012;7(10):1591-7.
- Peters B, Mölne J, Hadimeri H, Hadimeri U, Stegmayr B. Sixteen Gauge biopsy needles are better and safer than 18 Gauge in native and transplant kidney biopsies. Acta Radiol. 2017;58(2):240-248.
- Chunduri S, Whittier WL, Korbet SM. Adequacy and complication rates with 14- vs. 16-gauge automated needles in percutaneous renal biopsy of native kidneys. Semin Dial. 2015;28(2):11-4.
- Corapi KM, Chen JL, Balk EM, Gordon CE. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60(1):62-73.
- Whittier WL, Korbet SM. Who should perform the percutaneous renal biopsy: a nephrologist or radiologist? Semin Dial. 2014;27(3):243-5.
- Luciano RL, Moeckel GW. Update on the Native Kidney Biopsy: Core Curriculum 2019. Am J Kidney Dis. 2019;73(3):404-415.
- Mackinnon B, Fraser E, Simpson K, Fox JG, Geddes C. Is it necessary to stop antiplatelet agents before a native renal biopsy? Nephrol Dial Transplant. 2008;23(11):3566-70.
- Nayak-Rao S. Percutaneous native kidney biopsy in patients receiving antiplatelet agents- is it necessary to stop them routinely? Indian J Nephrol. 2015;25(3):129-32.
- Atwell TD, Smith RL, Hesley GK, Callstrom MR, Schleck CD, Harmsen WS, et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol. 2010;194(3):784-9.
- Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2):e326S-e350S.
- Hedges SJ, Dehoney SB, Hooper JS, Amanzadeh J, Busti AJ. Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol. 2007;3(3):138-53.
- Najafian B, Lusco MA, Alpers CE, Fogo AB. Approach to Kidney Biopsy: Core Curriculum 2022. Am J Kidney Dis. 2022;80(1):119-131.
- Mokhtar GA, Jallalah SM. Role of electron microscopy in evaluation of native kidney biopsy: a retrospective study of 273 cases. Iran J Kidney Dis. 2011;5(5):314-9.
- Collan Y, Hirsimäki P, Aho H, Wuorela M, Sundström J, Tertti R, et al. Value of electron microscopy in kidney biopsy diagnosis. Ultrastruct Pathol. 2005;29(6):461-8.
- Renal biopsy. Medline Plus. Available at http://www.nlm.nih.gov/medlineplus/ency/article/003907.htm. 2020 Apr 09; Accessed: May 2023.
- Montes D, Beamish C, Waheed S, Osman F, Maursetter L. What happens after the kidney biopsy? The findings nephrologists should know. BMC Nephrol. 2022 Jul 25;23(1):265.
- Habas E, Elhabash B, Rayani A, Turgman F and Tarsien R. Post-Percutaneous Renal Biopsy Observation Time; Single Center Experience. Austin J Nephrol Hypertens. 2016; 3(2): 1058.
- Alleyne G, Binagwaho A, Haines A, Jahan S, Nugent R, Rojhani A, Stuckler D; Lancet NCD Action Group. Embedding non-communicable diseases in the post-2015 development agenda. Lancet. 2013 Feb 16;381(9866):566-74.
- Ubara Y, Kawaguchi T, Nagasawa T, Miura K, Katsuno T, Morikawa T, et al; Committee of Practical Guide for Kidney Biopsy 2020. Kidney biopsy guidebook 2020 in Japan. Clin Exp Nephrol. 2021;25(4):325-364. doi: 10.1007/s10157-020-01986-6.
- Yuan CM, Jindal RM, Abbott KC. Biopsy: observation time after kidney biopsy: when to discharge? Nat Rev Nephrol. 2009;5(10):552-4.
- Maya ID, Allon M. Percutaneous renal biopsy: outpatient observation without hospitalization is safe. Semin Dial. 2009;22(4):458-61.
- Marwah DS, Korbet SM. Timing of complications in percutaneous renal biopsy: what is the optimal period of observation? Am J Kidney Dis. 1996;28(1):47-52.
- Whittier WL, Korbet SM. Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol. 2004;15(1):142-7.
- Fraser IR, Fairley KF. Renal biopsy as an outpatient procedure. Am J Kidney Dis. 1995;25(6):876-8.
- Oviasu E, Ugbodaga P. Evaluation of percutaneous renal biopsy as a day case procedure: experience from Nigeria. J Nephrol.;11(5):246-8.
- Murphy BF, MacIsaac A. Percutaneous renal biopsy as a day-patient procedure. Am J Kidney Dis. 1989;14(1):77.
- Bairy M, Beleed K, Webb AT, Bhandari S. Safety of outpatient kidney biopsy: one center's experience with 178 native kidney biopsies. Am J Kidney Dis. 2008;52(3):631-2.
- Kidney biopsy. National Institute of Diabetes and Digestive and Kidney Diseases. Available at https://www.niddk.nih.gov/health-information/diagnostic-tests/kidney-biopsy. 2015 November; Accessed: May, 2023.
- Poggio ED, McClelland RL, Blank KN, Hansen S, Bansal S, Bomback AS, et al. Systematic Review and Meta-Analysis of Native Kidney Biopsy Complications. Clin J Am Soc Nephrol. 2020;15(11):1595-1602.
- Altebarmakian VK, Guthinger WP, Yakub YN, Gutierrez OH, Linke CA. Percutaneous kidney biopsies. Complications and their management. Urology. 1981;18(2):118-22.
- Bolton WK. Nonhemorrhagic decrements in hematocrit values after percutaneous renal biopsy. JAMA. 1977;238(12):1266-8.
- Khajehdehi P, Junaid SM, Salinas-Madrigal L, Schmitz PG, Bastani B. Percutaneous renal biopsy in the 1990s: safety, value, and implications for early hospital discharge. Am J Kidney Dis. 1999;34(1):92-7.
- Ubara Y, Kawaguchi T, Nagasawa T, Miura K, Katsuno T, Morikawa T, Ishikawa E, et al; Committee of Practical Guide for Kidney Biopsy 2020. Kidney biopsy guidebook 2020 in Japan. Clin Exp Nephrol. 2021;25(4):325-364.
- Whittier WL, Sayeed K, Korbet SM. Clinical factors influencing the decision, to transfuse after percutaneous native kidney biopsy. Clin Kidney J. 2016;9(1):102-7.
- Alter AJ, Zimmerman S, Kirachaiwanich C. Computerized tomographic assessment of retroperitoneal hemorrhage after percutaneous renal biopsy. Arch Intern Med. 1980;140(10):1323-6.
- Montes D, Beamish C, Waheed S, Osman F, Maursetter L. What happens after the kidney biopsy? The findings nephrologists should know. BMC Nephrol. 2022;23(1):265.
- Waldo B, Korbet SM, Freimanis MG, Lewis EJ. The value of post-biopsy ultrasound in predicting complications after percutaneous renal biopsy of native kidneys. Nephrol Dial Transplant. 2009; 24(8):2433-9. doi: 10.1093/ndt/gfp073.
- Ishikawa E, Nomura S, Hamaguchi T, Obe T, Inoue-Kiyohara M, et al. Ultrasonography as a predictor of overt bleeding after renal biopsy. Clin Exp Nephrol. 2009;13(4):325-31.
- Niroshan V, Balagobi B, Brammah T, Weerasinghe N, Gowribahan T. Surgically managed acute Page kidney following renal biopsy-A case report. Int J Surg Case Rep. 2022;99:107641.
- Vaidya PN, Rathi BM, Finnigan NA. Page Kidney. [Updated 2023 Jan 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482486/: Accessed May 2023.
- Naranjo J, Villanego F, Cazorla JM, León C, Ledo MJ, García A, et al. Acute Renal Failure in Kidney Transplantation Due to Subcapsular Hematoma After a Renal Allograft Biopsy: Report of Two Cases and Literature Review. Transplant Proc. 2020;52(2):530-533.
- Lin SY, Chang CY, Lin CC, Hsu WH, Liu IW, Lin CD, et al. Complications of Outpatient and Inpatient Renal Biopsy: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2021;11(4):651.
- Rollino C, Garofalo G, Roccatello D, Sorrentino T, Sandrone M, Basolo B, et al. Colour-coded Doppler sonography in monitoring native kidney biopsies. Nephrol Dial Transplant. 1994;9(9):1260-3.
- Bergman SM, Frentz GD, Wallin JD. Ureteral obstruction due to blood clot following percutaneous renal biopsy: resolution with intraureteral streptokinase. J Urol. 1990;143(1):113-5.
- Harrison KL, Nghiem HV, Coldwell DM, Davis CL. Renal dysfunction due to an arteriovenous fistula in a transplant recipient. J Am Soc Nephrol. 1994;5 (6):1300-6.
- Ngoh CLY, Wee BBK, Wong WK. Lumbar Artery Bleed as a Complication of Percutaneous Renal Biopsy and a Proposed Workflow for Massive Bleeding. Case Rep Nephrol Dial. 2018;8(3):268-276.
- Yang CY, Lai MY, Lu CL, Tseng HS, Chiou HJ, Yang WC, et al. Timing of Doppler examination for the detection of arteriovenous fistula after percutaneous renal biopsy. J Clin Ultrasound. 2008;36(6):377-80.
- Lubomirova M, Krasteva R, Bogov B, Paskalev E. Incidence of A-V Fistulas after Renal Biopsy of Native and Transplanted Kidney - Two Centers Experience. Open Access Maced J Med Sci. 2015;3(2):241-4.
- Sosa-Barrios RH, Burguera V, Rodriguez-Mendiola N, Galeano C, Elias S, Ruiz-Roso G, et al. Arteriovenous fistulae after renal biopsy: diagnosis and outcomes using Doppler ultrasound assessment. BMC Nephrol. 2017;18(1):365.
- Sosa-Barrios RH, Burguera V, Rodriguez-Mendiola N, Galeano C, Elias S, Ruiz-Roso G, et al. Arteriovenous fistulae after renal biopsy: diagnosis and outcomes using Doppler ultrasound assessment. BMC Nephrol. 2017;18(1):365.
- Güneyli S, Gök M, Bozkaya H, Çınar C, Tizro A, Korkmaz M, et al. Endovascular management of iatrogenic renal arterial lesions and clinical outcomes. Diagn Interv Radiol. 2015;21(3):229-34.
- Sun HJ. Current Opinion for Hypertension in Renal Fibrosis. Adv Exp Med Biol. 2019;1165:37-47.
- Limwattana S, Rianthavorn P. Urinoma following Kidney Biopsy: A Case Report. Urol Int. 2015;95(2):246-8.
- Chahrour H, Chaaban A, Amado A, Hindi H, Harb A. Acute presentation of renal pseudoaneurysms in a patient with systemic lupus erythematosus after percutaneous renal biopsy. Radiol Case Rep. 2023;18(9):2935-2938.
- Sood P, Cherikh WS, Toll AE, Mehta RB, Hariharan S. Kidney allograft rejection: Diagnosis and treatment practices in USA- A UNOS survey. Clin Transplant. 2021;35(4):e14225.
- Rush D, Nickerson P, Gough J, McKenna R, Grimm P, Cheang M, et al. Beneficial effects of treatment of early subclinical rejection: a randomized study. J Am Soc Nephrol. 1998;9(11):2129-34.
- Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS, Hobbs FD. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One. 2016;11(7):e0158765.
- Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12(1):7-11.
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-733.
- Zhang T, Yang X, Zhang M, Zhou W, Jin Y, Zhou H, et al. Effects of receiving renal biopsy on the prognosis of chronic kidney disease patients with impaired renal function. BMC Nephrol. 2023;24(1):56.
- Joseph AJ, Compton SP, Holmes LH, Annand A, Self SE, et al. Utility of percutaneous renal biopsy in chronic kidney disease. Nephrology (Carlton). 2010;15(5):544-8.
- Tomson CR. Indications for renal biopsy in chronic kidney disease. Clin Med (Lond). 2003;3(6):513-7.
- Maripuri S, Penson DF, Ikizler TA, Cavanaugh KL. Outpatient versus inpatient observation after percutaneous native kidney biopsy: a cost minimization study. Am J Nephrol. 2011;34(1):64-70.
- irks JH, de Zeeuw D, Agarwal SK, Atkins RC, Correa-Rotter R, D’Amico G, et al. Prevention of chronic kidney and vascular disease: toward global health equity--the Bellagio 2004 Declaration. Kidney Int Suppl. 2005;(98):S1-6.
- Akinsola W, Odesanmi WO, Ogunniyi JO, Ladipo GO. Diseases causing chronic renal failure in Nigerians--a prospective study of 100 cases. Afr J Med Med Sci. 1989;18(2):131-7.
- Okani CO, Ekrikpo UE, Okolo CA, Asinobi AO, Salako B, Akang EE. Is the art of renal biopsy on the decline in Nigeria? Ann Ib Postgrad Med. 2014;12(1):38-41.
- Chesney DS, Brouhard BH, Cunningham RJ. Safety and cost effectiveness of pediatric percutaneous renal biopsy. Pediatr Nephrol. 1996;10(4):493-5.
- Dutta R, Okhunov Z, Vernez SL, Kaler K, Gulati AT, Youssef RF, et al. Cost Comparisons Between Different Techniques of Percutaneous Renal Biopsy for Small Renal Masses. J Endourol. 2016;30(1):28-33.
- Dattani R, Corbett RW, Galliford J, Hill P, Cairns T, Cook HT, Roufosse C, et al. The Effect of Kidney Biopsy on Glomerular Filtration Rate: A Frequent Patient Concern. Am J Nephrol. 2020:1-4.
- Giannico G, Fogo AB. Lupus nephritis: is the kidney biopsy currently necessary in the management of lupus nephritis? Clin J Am Soc Nephrol. 2013;8(1):138-45.
- Habas E, Khan F. Lupus Nephritis: Pathogenesis and Treatment Update Review. Open Science Journal. 2021;6(1).1-16.
- Schnuelle P. Renal Biopsy for Diagnosis in Kidney Disease: Indication, Technique, and Safety. J Clin Med. 2023;12(19):6424.
- Naik RH, Shawar SH. Renal Transplantation Rejection. [Updated 2023 Feb 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553074/. Accessed Dec 2023.
- Williams WW, Taheri D, Tolkoff-Rubin N, Colvin RB. Clinical role of the renal transplant biopsy. Nat Rev Nephrol. 2012;8(2):110-21.
