Full HTML
Metformin – A New Frontier in Skin Cancer Pharmacotherapy
Philip O. Ogbeye1,4, Ebisindor V. Awala2,4, Darlington A. Dovieme1,4, Oyeintonbara Miediegha1,4, Samuel J. Bunu1,3,4
Author Affiliation
1 Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa State, Nigeria
2 Department of Healthcare Informatics, University of the Potomac, USA
3 Drug Analysis and Research Center, Ebisamdex Global Ventures Ltd, Yenagoa, Bayelsa State, Nigeria
4 Pharmacist
Abstract
Metformin, a widely prescribed biguanide for type 2 diabetes, has emerged as a promising candidate in skin cancer therapy due to its diverse anticancer mechanisms. Beyond its glucose-lowering effects, metformin inhibits key oncogenic pathways, including the PI3K/AKT/mTOR and insulin/IGF-1 signaling pathways, activates AMP-activated protein kinase, and disrupts mitochondrial complex I function. These mechanisms are presumed to contribute to metformin's antiproliferative, pro-apoptotic, and anti-inflammatory effects, potentially reducing tumor growth and metastasis in melanoma and non-melanoma skin cancers. Predictive molecular docking studies reveal that metformin interacts with critical proteins in melanoma pathophysiology. Against PI3K/mTOR (PDB: 5OQ4), PTPN2 (PDB: 7UAD), and TRIP13 (PDB: 5VQA), metformin exhibited docking scores of -4.4, -4.6, and -5.6 kcal/mol, respectively, interacting via hydrogen bonding with residues such as ASP-836, ASP-964 (5OQ4), ASP-50 (7UAD), and SER-187, SER-138 (5VQA). Compared to standard inhibitors, PQR309 (-9.4 kcal/mol), ABBV-CLS-484 (-7.5 kcal/mol), and ATP (-10.8 kcal/mol), metformin displayed moderate binding affinity, suggesting potential but weaker inhibition of these targets. Preclinical and clinical studies support metformin's potential to reduce skin cancer risk, particularly in diabetic patients. However, challenges regarding bioavailability, optimal dosing, and patient selection persist, necessitating further investigation. Therefore, given its affordability, safety, and multitargeted action, metformin represents an attractive candidate for repurposing in skin cancer pharmacotherapy. Focusing future research on optimizing its therapeutic application, refining drug combinations, and identifying biomarkers would enhance clinical outcomes.
DOI: 10.63475/yjm.v4i1.0091
Keywords: Metformin, Melanoma, Ultraviolet Radiation
Pages: 65-78
View: 20
Download: 27
DOI URL: https://doi.org/10.63475/yjm.v4i1.0091
Publish Date: 22-05-2025
Full Text
The skin, the body's largest organ, forms the external protective layer that shields against ultraviolet radiation and harmful agents, while also playing a key role in temperature regulation. It has three primary layers: the epidermis, dermis, and hypodermis. [1] Skin cancer remains one of the most prevalent malignancies worldwide, with its incidence steadily rising due to factors such as increased ultraviolet (UV) radiation exposure, genetic predisposition, and environmental influences. [2] While conventional treatment strategies, including surgery, chemotherapy, radiation therapy, and immunotherapy, have shown effectiveness, they are often associated with significant limitations, such as toxicity, resistance, and high costs. [3, 4] Consequently, there is a growing need to explore alternative pharmacological approaches that offer improved safety, efficacy, and accessibility. [4]
Metformin (Figure 1), a widely used biguanide medication, is valued for its safety and affordability. For over six decades, it has been a first-line treatment for type 2 diabetes due to its remarkable ability to lower plasma glucose levels. Over time, additional applications of metformin have been discovered, with its benefits extending to various human cancers, obesity, liver, cardiovascular, and renal diseases, as well as aging diseases through multiple signaling pathways. [5] While traditionally known for its glucose-lowering effects, emerging research suggests that metformin may have therapeutic benefits in reducing the risk and progression of various cancers, including melanoma skin cancer (MSC) and non-melanoma skin cancer (NMSC). MSC and NMSC are among the most common cancers globally, with rising incidence and mortality rates, particularly in populations with lighter skin tones. [6] Skin cancer accounts for one-third of all diagnosed cancers worldwide, with NMSCs being the most frequently occurring type. [7]
Metformin (1,1-dimethyl biguanide), a biguanide oral antidiabetic agent, has been a cornerstone in the treatment of type 2 diabetes mellitus (T2DM) for many years. [8, 9] Its origin traces back to the traditional European herbal medicine, Galega officinalis. Galega officinalis, commonly known as goat’s rue, French lilac, Italian fitch, Spanish sainfoin, or professor’s herb, belongs to the Fabaceae family and is the plant from which metformin is derived. Medieval herbalists traditionally used it to treat polyuria (excessive urination), a hallmark symptom of diabetes. [10-12] In the mid-19th century, chemical studies of Galega officinalis showed that it contains high levels of guanidine and similar compounds. Later, in 1918, researchers discovered that guanidine could reduce blood glucose levels in animals. [13]
Metformin was first synthesized in 1922 and tested in animal studies for its ability to lower blood glucose levels. [15] The synthesis of metformin in 1922 by Werner and Bell was built upon earlier chemical discoveries that aligned with its herbal origins. Its foundation can be traced back to Adolph Strecker’s work in the mid-19th century on guanidine synthesis, followed by Bernhard Rathke’s research in 1879, which led to the fusion of two guanidines to form biguanide. [16, 17] Although metformin was much less toxic compared to mono- and diguanidines, [12] its relatively mild hypoglycemic effects at high doses caused it to be largely disregarded as a potential treatment. As a result, neither biguanides nor other guanidine-based compounds were pursued for diabetes treatment and were largely forgotten in the following decade. [18] However, interest in metformin resurfaced through research on antimalarial drugs, particularly the development of the guanidine-based compound proguanil (Paludrine), which was later modified into metformin. In the 1940s, clinical studies by Eusebio Garcia in the Philippines demonstrated metformin’s effectiveness in treating influenza, where it was marketed as flumamine, and its hypoglycemic properties were also observed. [19, 20]
.png)
Figure 1: Structure of metformin hydrochloride; 2D and 3D. From “Metformin HCl,” by World Biochemicals Industries Ltd., n.d. (https://www.wbcil.com/organic-molecules/metformin-hcl/). [14]
The glucose-lowering effects of flumamine were further studied in animal models and clinical trials by Jean Sterne, a French physician who, in 1957, was the first to report its use for diabetes treatment. [21] Jean Sterne, collaborating with his pharmacist colleague Denis Duval, conducted a comprehensive research program to study the pharmacodynamics of guanidine-derived compounds, such as metformin and phenformin, in both healthy and diabetic animal models. Unintentionally, they replicated and expanded on 1920s research, rediscovering issues like high-dose limitations, modest glucose-lowering effects, and notable toxicity. However, they identified metformin as the most promising candidate for clinical trials in diabetes treatment due to its effective glucose-lowering properties, minimal side effects in animal models, and prior documented use of flumamine in humans. [16] Despite Sterne’s findings, metformin initially received little attention, as it was considered less potent than other biguanides like phenformin and buformin in lowering blood sugar. [22, 23] However, by the late 1970s, phenformin and buformin were withdrawn due to their high risk of lactic acidosis. [12] The occurrence of lactic acidosis in metformin users was significantly lower, with most cases linked to improper use in patients who had chronic renal impairment or acute kidney disease, despite contraindications. [24-26] However, metformin's credibility was undermined due to its association with other biguanides, placing it at risk of discontinuation. Nevertheless, pharmacokinetic and pharmacodynamic studies in Europe during the 1980s highlighted metformin’s effectiveness in reducing insulin resistance and managing hyperglycemia in adults without causing weight gain or increasing the risk of hypoglycemia. [12, 26, 28] In the United States, skepticism toward metformin persisted due to adverse experiences with other biguanides, leading to initial reluctance from the FDA to approve its use. However, following persistent efforts by Dr. Gerard Daniel, a thorough review of existing research was conducted, leading to clinical trials. As a result, metformin was approved for use in the U.S. in 1995. [29, 30] In 1998, further evidence reinforced its role as the first-line treatment for type 2 diabetes, particularly highlighting its long-term cardiovascular benefits. Several decades after its introduction as a diabetes treatment, metformin, a dimethylbiguanide derived from the guanidine in Galega officinalis, has become the most widely prescribed oral hypoglycemic drug worldwide, with potential applications beyond diabetes, including cancer treatment. [12, 26, 28]
The interest in metformin’s anticancer potential stems from epidemiological studies that indicate a reduced cancer incidence and improved prognosis among diabetic patients treated with the drug. [31] Research has shown that metformin exerts its antineoplastic effects primarily by activating the AMP-activated protein kinase (AMPK) pathway, leading to the inhibition of the mechanistic target of rapamycin (mTOR), a key regulator of cell growth and proliferation. [32] Additionally, metformin disrupts cancer metabolism by reducing mitochondrial oxidative phosphorylation, thereby inducing an energy crisis in rapidly dividing tumor cells. [33] The potential repurposing of metformin for skin cancer therapy presents a promising advancement in cancer pharmacotherapy. Given its well-documented safety profile and metabolic regulatory properties, metformin offers a cost-effective and readily available treatment approach that may serve as a valuable adjunct or primary agent to current skin cancer management strategies. [34] Therefore, this article explores the emerging evidence supporting Metformin’s potential application in skin cancer therapy. Specifically, the biological mechanisms by which Metformin influences cancer cell biology, preclinical and clinical studies, potential molecular binding targets, and how it can be integrated into current treatment strategies as a new frontier in skin cancer pharmacotherapy.
The pathogenesis of skin cancer is multifactorial, with ultraviolet radiation (UVR) from sunlight being the primary cause of both malignant melanoma (MSC) and non-melanoma skin cancer (NMSC). [35] MSC arises from the uncontrolled proliferation of melanocytes, which are pigment-producing cells originating from the neural crest. These cells generate melanin, the brown pigment that determines skin color and provides protection against harmful solar radiation. [36] The development of malignant skin cancer, or MSC, is influenced by both biological and environmental factors. A critical biological factor is genetic mutations in the cyclin-dependent kinase inhibitor 2A (CDKN2A) gene, which encodes two tumor suppressor proteins, ARF (p14ARF) and p16INK4A, essential for regulating the cell cycle. Disruptions in these proteins due to mutations can lead to the onset of skin cancers like MSC. [37] Ultraviolet (UV) radiation is a major environmental risk factor for skin cancer, contributing to melanoma development through mechanisms such as DNA and RNA damage, genetic mutations, and the inactivation of the p53 protein, which normally promotes cell death (Figure 2). UV radiation has a stronger association with melanoma risk compared to basal cell carcinoma (BCC) or squamous cell carcinoma (SCC). [38] NMSCs, the most common human cancers, consist of two primary types: BCC and SCC, both originating from epidermal keratinocytes. [39, 40] BCC, the most prevalent malignant neoplasm in humans, develops from the outermost epidermal layer, while SCC arises from keratinocytes in squamous and non-squamous epithelial tissues. [41, 42]
.png)
Figure 2: Pathophysiology of skin cancer – melanoma. [43]
The malignant transformation of melanocytes occurs through both physiological processes and UV-induced mechanisms. Under normal conditions, keratinocytes promote melanocyte proliferation by secreting melanocyte-stimulating hormone (MSH), which binds to the melanocortin 1 receptor (MC1R). UV-A radiation contributes to this transformation via two main pathways: first, by directly causing mutations in proto-oncogenes and tumor suppressor genes (e.g., TP53, NF1, PTEN), converting normal melanocytes into cancerous cells; and second, by transforming melanocytes into benign nevi, 80% of which carry the BRAFV600E mutation. These nevi often remain inactive for years, partly due to immune surveillance. However, further UV exposure can induce additional mutations, such as in TERT and CDKN2A, driving their progression into malignant forms. [43]
Exposure to environmental carcinogens, particularly ultraviolet or UV radiation, induces DNA damage, resulting in mutations within the p53 gene, observed in approximately 90% of SCC and nearly 50% of BCC. These mutations impair the function of the p53 tumor suppressor protein, ultimately promoting the development of NMSC. [44] Enzymes/proteins and genes, such as the Thyroid Hormone Receptor Interacting Protein-13 (TRIP13), and the matrix remodeling associated-7 (MXRA7), have also been perceived to play a role in skin cancers, especially melanoma. [45-46] Diabetic patients face a higher risk of developing cancers compared to healthy individuals, partly because of elevated levels of circulating growth factors like insulin and insulin-like growth factors 1 and 2 (IGF-1 and IGF-2). [47] IGF plays a role in regulating epidermal cell proliferation. In diabetic patients, increased serum levels of insulin and IGFs are linked to enhanced cellular proliferation and the activation of oncogenic epidermal growth factor receptors, resulting in mitogenic and antiapoptotic effects that drive the transformation of cells into malignant forms. [48]
A variety of drug classes are currently employed in the treatment of melanoma and other related skin cancers, with the choice of therapy largely influenced by factors such as disease stage, genetic mutations, and overall progression. These therapeutic options include immunotherapy, which harnesses the body's immune system to fight cancer. Key agents in this category are checkpoint inhibitors, such as PD-1 inhibitors (Pembrolizumab, Nivolumab), which help restore immune system activity against tumor cells, and CTLA-4 inhibitors (Ipilimumab), which enhance T-cell function. Also, Interleukin-2 (IL-2) (Aldesleukin) is sometimes used to stimulate immune responses in advanced melanoma cases. [49, 50] Another important class is targeted therapeutic agents, which focus on specific genetic mutations present in melanoma cells. For BRAF-mutant melanoma, BRAF inhibitors (Vemurafenib, Dabrafenib, Encorafenib) are used, often in combination with MEK inhibitors (Trametinib, Cobimetinib, Binimetinib) to enhance efficacy and reduce resistance. Meanwhile, in cases of KIT-mutant melanoma, KIT inhibitors such as Imatinib and Nilotinib may be effective. [51, 52]
Traditional chemotherapy, though less commonly used due to lower response rates, remains an option in certain cases. This includes Dacarbazine (DTIC), which is FDA-approved for melanoma, as well as Temozolomide, an oral alternative. Also, combination regimens such as Paclitaxel and Carboplatin may be considered in specific scenarios. [53-55] A more recent advancement in melanoma treatment is oncolytic virus therapy, where genetically engineered viruses selectively infect and destroy cancer cells. One such therapy is Talimogene laherparepvec (T-VEC), a modified herpes simplex virus designed to attack melanoma cells while also stimulating an anti-tumor immune response. [56] These treatment options, often used in combination or sequentially, have significantly improved the prognosis and survival rates of patients with melanoma, particularly in advanced or metastatic stages.
Metformin lowers both fasting and post-meal glucose levels, mainly by curbing excessive liver glucose production through the inhibition of gluconeogenesis. This is achieved through the inhibition of mitochondrial complex 1, which alters cellular energy balance by reducing ATP production. [33] Gluconeogenesis is an energetically costly process, requiring 6 ATP equivalents per molecule of glucose synthesized, and a reduction in ATP production impairs the anabolic process. [57] This reduction in ATP also increases the AMP: ATP ratios and the ADP: ATP ratios, activating AMPK (AMP-activated protein kinase), a key regulator of metabolism. [58] The primary pharmacological impact of AMPK activation in the liver involves promoting fatty acid oxidation while suppressing the synthesis of cholesterol and triglycerides.
Metformin may also enhance glucose uptake and insulin signaling, reduce fatty acid and triglyceride synthesis, and promote fatty acid β-oxidation. [59] Additionally, it can improve glucose utilization in peripheral tissues and potentially decrease intestinal glucose absorption, preventing rapid spikes in blood glucose levels. [60] Metformin improves glucose uptake by boosting the expression of insulin receptor substrates 1 and 2 (IRS-1 and 2) and facilitating the movement of glucose transporters, such as glucose transporter 1 (GLUT-1), to the plasma membrane in both liver and skeletal muscle cells. [47]
Metformin passes through the urine unaltered since it is not metabolized by the liver or other tissues. [61] The primary route of elimination is the kidneys' active tubular secretion. The medication is absorbed in the kidney, liver, and intestines through Organic Cation Transporters (OCTs). Individual differences in metformin's pharmacokinetics have been observed. [62] Intestinal absorption of metformin may be facilitated by the plasma membrane monoamine transporter (PMAT), while OCT1 and possibly OCT3 primarily mediate hepatic uptake. Additionally, metformin serves as a substrate for human multidrug and toxin extrusion transporters MATE1 and MATE2-K, which may contribute to its excretion from the liver and kidneys. However, the role of MATE1 in hepatic secretion remains unclear. Metformin uptake into renal epithelial cells is largely mediated by OCT2, located in the basolateral membrane of renal tubules. Renal excretion is facilitated by MATE1 and MATE2-K, which are expressed in renal proximal tubule cells. PMAT and OCT1 may also play a role in metformin reabsorption within the renal tubules. [63] Genetic polymorphisms influence metformin's pharmacokinetics and pharmacological responses, while drug interactions that inhibit metformin transporters are clinically significant. Recent studies on drug-drug interactions suggest that proton pump inhibitors, oral antidiabetic drugs, and cimetidine may reduce metformin absorption. Additionally, certain tyrosine kinase inhibitors may interact with metformin via transporters, potentially affecting its toxicity, efficacy, and overall disposition. [64]
Absorption: Metformin is administered orally in doses ranging from 500 mg two or three times daily, up to a maximum of 2,550 mg per day or 35 mg/kg/day. Peak plasma levels (Cmax) are achieved within 1–3 hours for immediate-release metformin and 4–8 hours for extended-release formulations. [65-66] Metformin has a 40–60% oral bioavailability and a 6-hour gastrointestinal absorption period. [62]
Distribution: Metformin rapidly enters the body but takes longer to reach deeper compartments. It accumulates in various organs, including the kidneys, salivary glands, stomach, duodenum, and esophagus. Over 24 hours, it increases the blood-to-plasma concentration ratio without binding to plasma proteins. [62]
Metabolism and excretion: Metformin is not metabolized but is eliminated from the body through tubular secretion and excreted unchanged in the urine. [64] It is quickly cleared by the kidneys, with a plasma elimination half-life of 1.5 to 4.5 hours following intravenous injection and 2.0 to 6.0 hours after oral administration in healthy individuals. [67] It has a renal clearance of 510 ± 120 mL/min. Its high clearance is attributed to its low molecular weight, the presence of renal transporters, and low lipid solubility. [68] A decline in renal function is directly associated with reduced metformin clearance. The medication should not be initiated in patients over 80 years old and is contraindicated in individuals with elevated blood creatinine levels or impaired renal clearance. [68]
Metformin mediates its anticancer effects via several direct and indirect mechanisms (Figure 3), including inhibition of the mTOR (mammalian target of rapamycin) pathway, suppression of IGF-1 signaling, and regulation of cell cycle progression and apoptosis. These mechanisms collectively contribute to reduced cancer cell proliferation, metabolic stress, and enhanced sensitivity to therapy. [69]
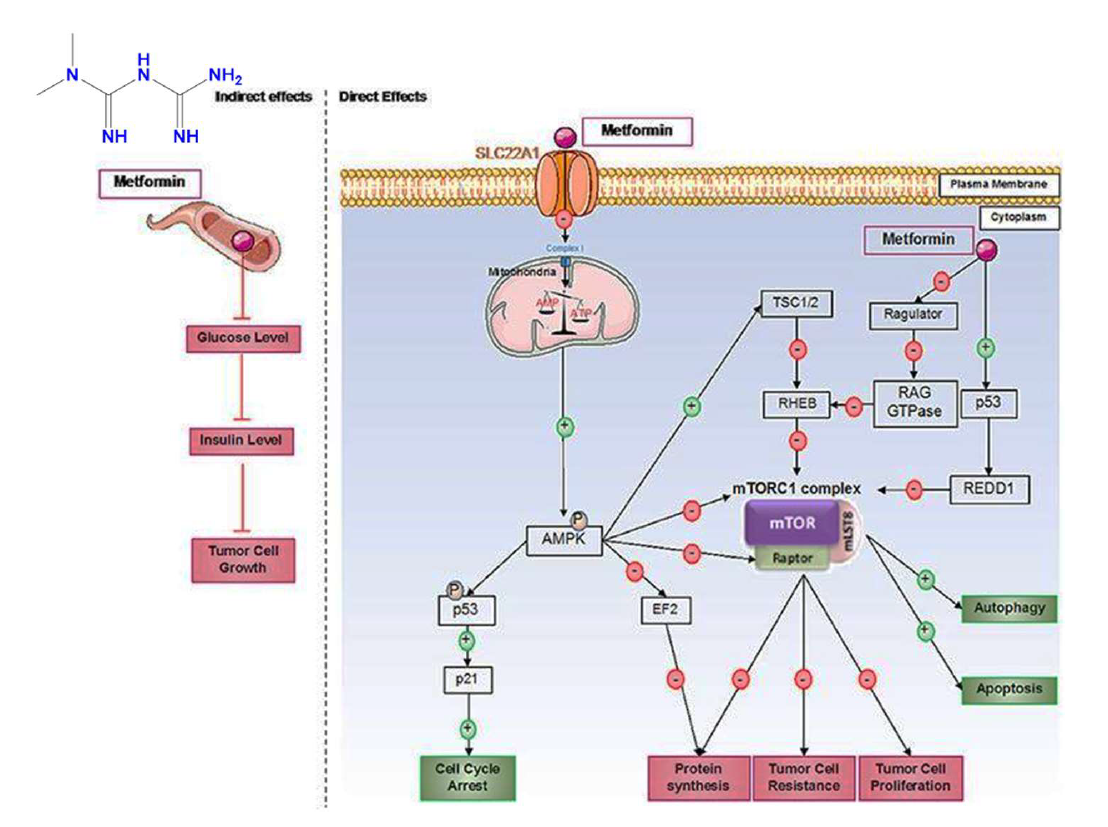
Figure 3: Direct and indirect mechanisms of action of metformin in cancer cells. [47]
Metformin exhibits antitumor effects through both indirect and direct mechanisms. Indirectly, it reduces blood glucose levels, leading to decreased insulin levels, which is significant since insulin can act as a growth factor for tumor cells. Directly, metformin operates through AMPK-dependent and independent pathways. It primarily suppresses the mTORC1 pathway, a critical protein complex involved in processes like protein synthesis and cell proliferation, which also plays a role in tumor cell resistance to treatments. Furthermore, metformin induces cell cycle arrest by activating p53. [47]
Inhibition of mitochondrial complex I
Metformin has been shown to inhibit mitochondrial complex I (NADH dehydrogenase) in cancer cells, thereby reducing tumorigenesis. By impairing mitochondrial respiration, metformin disrupts cellular energy metabolism, rendering cancer cells more susceptible to metabolic stress and apoptosis. Several epidemiological and preclinical studies suggest that this antidiabetic drug may play a role in cancer prevention and suppression. However, the exact mechanisms underlying its anticancer effects remain incompletely understood. A pivotal study by Wheaton et al. demonstrated that metformin inhibits mitochondrial complex I activity and cellular respiration in human cancer cells (Figure 6). [70] In glucose-rich conditions, metformin inhibited cell proliferation, whereas under glucose-deprived conditions, it induced cell death, indicating that cancer cells shift to a glycolysis-dependent survival mechanism when mitochondrial respiration is impaired. Additionally, metformin was found to reduce the hypoxic activation of hypoxia-inducible factor-1 (HIF-1), a key transcription factor involved in cancer progression. Notably, in vivo experiments further confirmed that metformin suppressed tumor growth in control cancer cells in mice. These findings collectively underscore that metformin’s anticancer activity is cell-autonomous and dependent on its inhibition of mitochondrial complex I. [70]
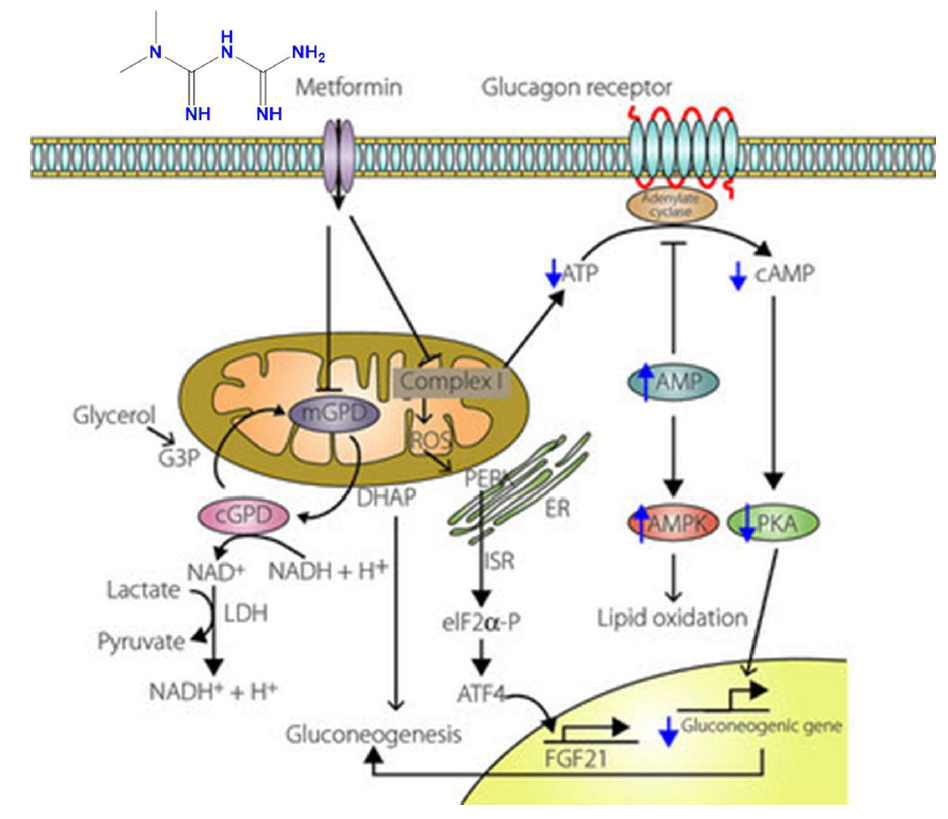
Figure 4: Metformin and mitochondrial complex I [71]
Metformin inhibits mitochondrial complex I, mitochondrial shuttle, and glucagon signaling, leading to reduced mitochondrial complex I activity. This decreases ATP levels and increases AMP, activating AMP-activated protein kinase (AMPK). The inhibition of complex I also increases mitochondrial ROS production, triggering the integrated stress response (ISR) via activation of double-stranded RNA-activated protein kinase-like ER kinase, resulting in eIF2α phosphorylation and ATF4 induction. ATF4 stimulates fibroblast growth factor 21 (FGF21) production. Metformin also inhibits mitochondrial glycerophosphate dehydrogenase (mGPD), blocking gluconeogenesis from glycerol. This leads to decreased NAD+ levels, causing lactate accumulation. Increased AMP further inhibits adenylate cyclase, reducing cAMP and attenuating glucagon-induced gluconeogenesis. [71]
AMP-activated protein kinase activation
Metformin exerts its anticancer effects, in part, through the activation of AMP-activated protein kinase (AMPK), a central cellular energy sensor. Activation of AMPK leads to inhibition of the mechanistic target of rapamycin (mTOR) pathway, a key regulator of cell growth, proliferation, and survival. The downregulation of mTOR signaling can suppress tumor progression and promote apoptosis in cancer cells. Although the precise mechanisms by which metformin activates AMPK remain under investigation, evidence suggests that even low concentrations, such as those reaching peripheral tissues like the skin, can enhance AMPK activity through phosphorylation of the AMPKα subunit at threonine 172 (Thr-172). These low levels facilitate the formation of the AMPK αβγ complex, promoting phosphorylation via liver kinase B1 (LKB1) and reducing dephosphorylation by protein phosphatase 2C (PP2C) (Figure 5). The relevance of this mechanism in skin cancer lies in the high metabolic demands of rapidly proliferating tumor cells. By activating AMPK and subsequently downregulating mTOR, metformin may shift the metabolic balance of skin cancer cells toward energy stress, thereby reducing their viability and growth potential. These findings highlight metformin’s potential as an adjunct or repurposed agent in the management of skin malignancies, particularly in cancers that display dysregulated mTOR signaling. [72]
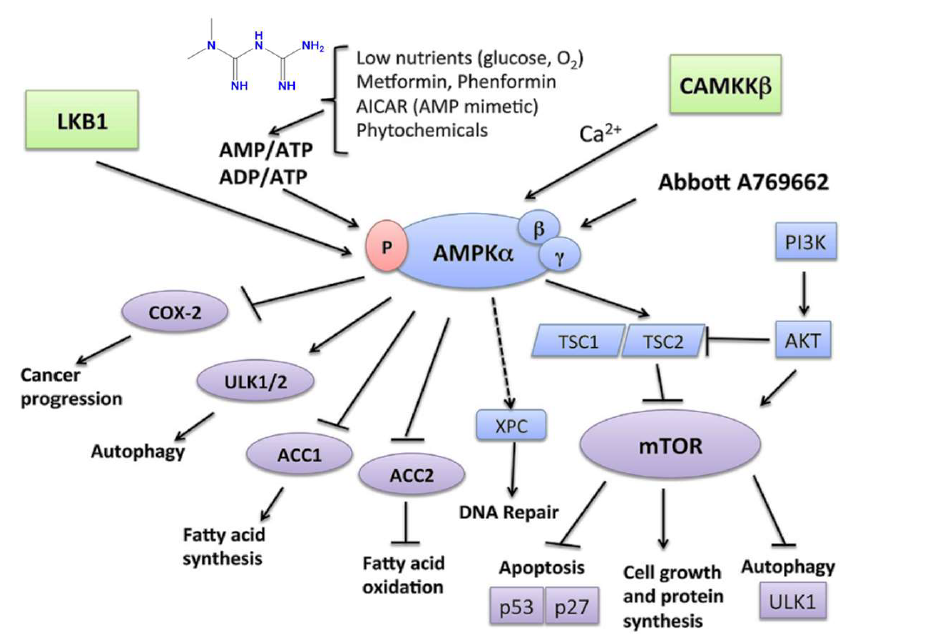
Figure 5. Association of metformin and AMPKα cancer progression. [73]
AMPK plays a vital role in tumor suppression through its regulatory functions. It is activated when cellular AMP/ATP or ADP/ATP ratios rise due to physiological stresses such as hypoglycemia or hypoxemia, leading to the activation of LKB1. Metformin and phenformin can replicate these stress conditions and activate AMPK in an LKB1-dependent manner. Additionally, CaMKKβ activates AMPK in response to increased calcium levels. Once activated, AMPK enhances catabolic pathways, such as fatty acid oxidation, by phosphorylating and inactivating acetyl-CoA carboxylase (ACC2), while inhibiting anabolic pathways like fatty acid synthesis, mediated by ACC1. A key mechanism of AMPK involves the Tuberous Sclerosis Complex 1 and 2 (TSC1/TSC2 complex), which downregulates mTOR, a pathway also influenced by PI3K-AKT and Ras-Raf-MEK-ERK signaling. mTOR suppresses apoptosis by affecting tumor suppressors like p53 and p27 and inhibits autophagy by repressing UNC-51-like kinase 1 (ULK1) and ULK2. AMPK counteracts these mTOR effects, promoting apoptosis and autophagy-mediated cell death. Independent of mTOR, AMPK phosphorylates and activates ULK1 and ULK2, further inducing autophagy. Moreover, AMPK downregulates cyclooxygenase (COX)-2 expression, which is linked to the progression of certain cancers and inflammatory diseases. AMPK is also crucial for the expression of xeroderma pigmentosum C (XPC), aiding DNA repair following UV-induced damage. [73]
Insulin/IGF-IR signaling pathway
Metformin, widely recognized for its glucose-lowering effects, may inhibit tumor growth by restricting glucose availability to cancer cells. Glucose is a critical energy source for rapidly proliferating tumors. [74] In the context of skin cancer, this mechanism is particularly relevant, as altered metabolic pathways and growth factor signaling contribute significantly to UV-induced carcinogenesis. Insulin and insulin-like growth factor 1 (IGF-1) function as key mitogenic signals that enhance cell survival and proliferation, processes that are closely linked to skin cancer development. [75-77] Their action is mediated through the insulin receptor (IR) and insulin-like growth factor 1 receptor (IGF-1R), which are expressed on keratinocytes and various skin tumor cells. [78] Upon activation, these receptors initiate oncogenic signaling cascades such as Ras/Raf/MEK/ERK and PI3K/Akt/mTORC1, which promote epidermal cell proliferation and resistance to apoptosis. [79-81] Hyperinsulinemia, a common feature in insulin-resistant states, has also been shown to elevate circulating bioactive IGF-1 by suppressing insulin-like growth factor-binding proteins (IGFBPs), resulting in heightened IGF-1R activity. [82] Metformin’s ability to lower systemic insulin and IGF-1 levels may therefore play a protective role by reducing IGF-1R-driven mitogenic signaling in the skin, ultimately helping to prevent or slow the progression of non-melanoma skin cancers. [83]
PI3K/AKT/mTOR pathway
Chronic exposure to ultraviolet (UV) radiation, especially UVA, is a key factor in skin aging and carcinogenesis. It promotes oxidative stress, mitochondrial dysfunction, and DNA damage, leading to cellular senescence and an increased risk of skin cancer. [84] In an in vitro and in vivo study by Chen et al. (2022), metformin treatment was shown to reverse UVA-induced cellular aging in human foreskin fibroblasts and a mouse model. The observed benefits were linked to suppression of the PI3K/AKT/mTOR pathway, inhibition of mitophagy, and reduction in mitochondrial oxidative stress. Notably, these pathways are not only implicated in photoaging but also play crucial roles in UV-induced tumorigenesis, as chronic activation can promote unchecked cell proliferation and resistance to apoptosis. By downregulating this pathway and restoring mitochondrial homeostasis, metformin may help maintain epidermal and dermal integrity, prevent premature senescence, and reduce the risk of UV-related skin malignancies, such as non-melanoma skin cancer (NMSC). [85]
UVA exposure increases mitochondrial ROS levels and lowers mitochondrial membrane potential, initiating mitophagy and resulting in DNA damage. It also activates the PI3K/AKT/mTOR signaling pathway, contributing to cell senescence. In contrast, metformin prevents mitochondrial damage, suppresses mitophagy, and inhibits the PI3K/AKT/mTOR signaling pathway in response to UVA-induced photoaging. [85]
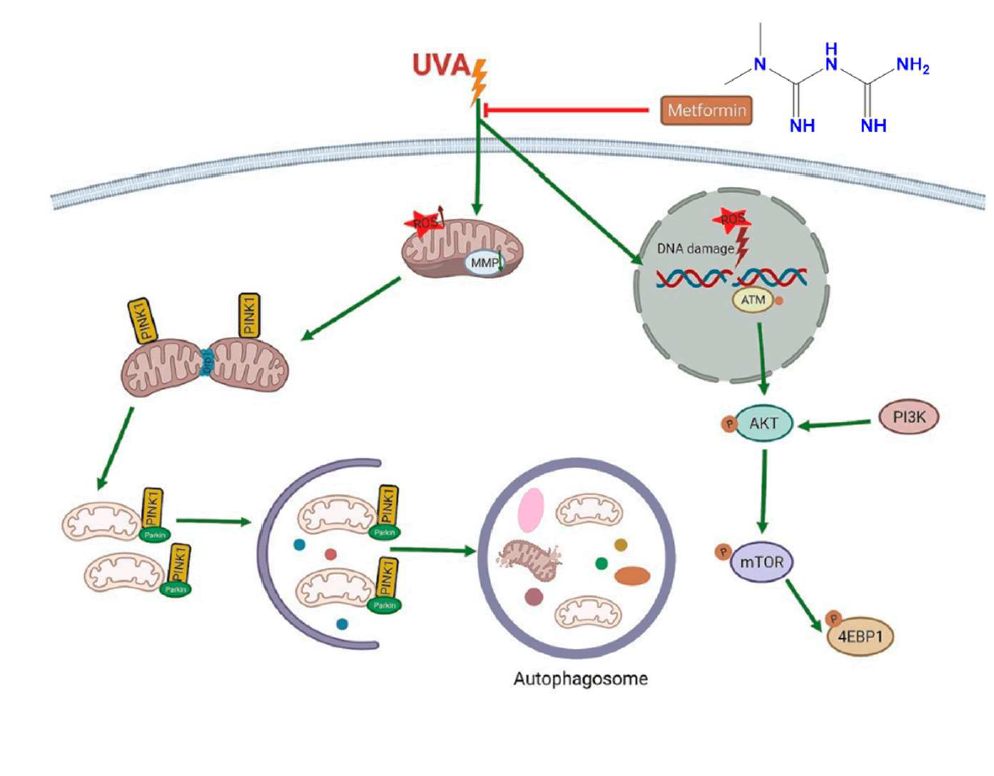
Figure 6. PI3K/AKT/mTOR pathway relationship with metformin and skin cancer. [85]
Table 1. Molecular docking scores: metformin with different proteins implicated in melanoma.
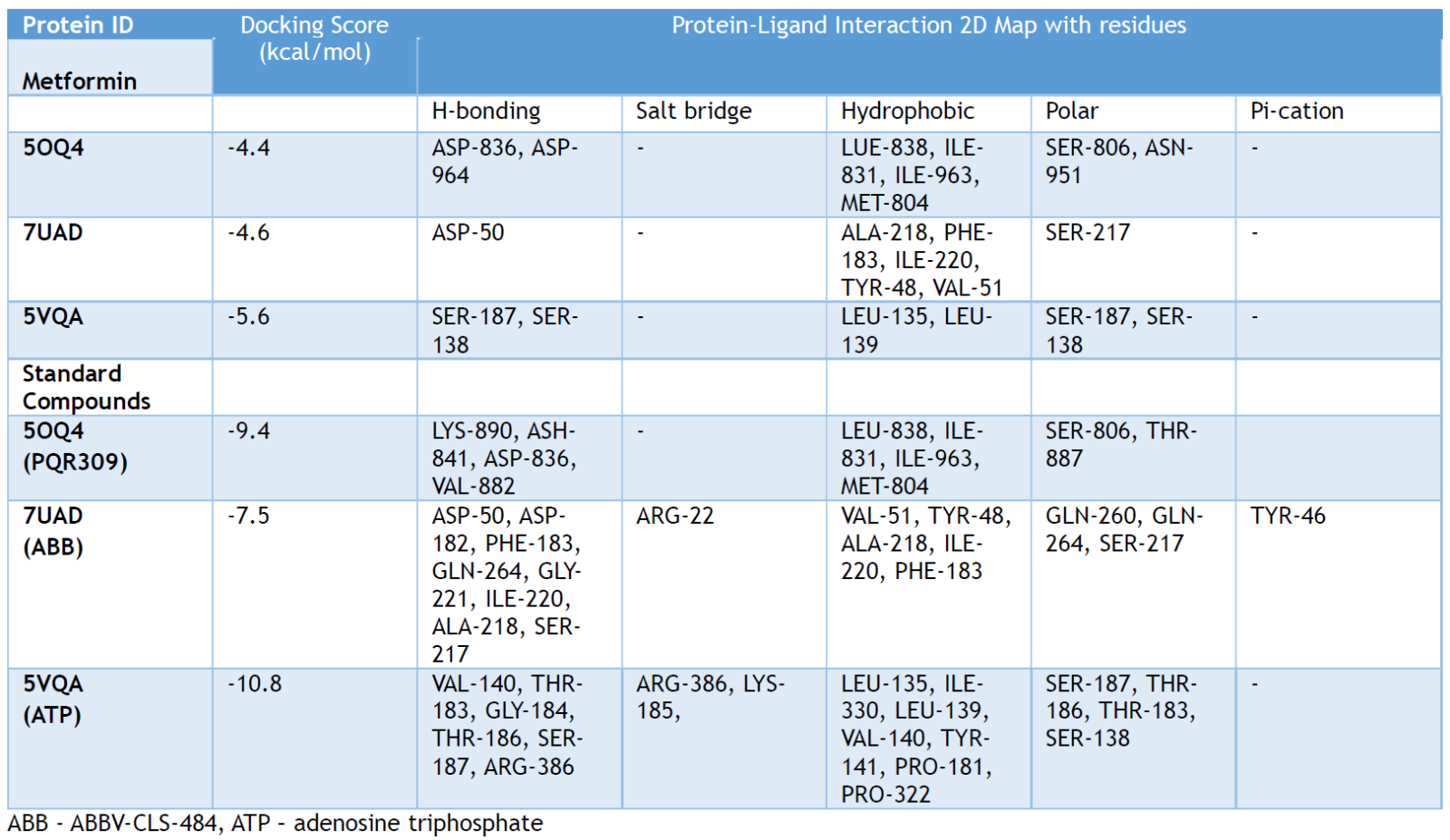
A preliminary molecular docking, modeling, and potential target prediction study was performed on three proteins reported to be associated with skin cancer. The 3D structure of pan-Class I PI3K/mTOR Inhibitor co-crystalized with PQR309 - a potent, brain-penetrant (PDB ID: 5OQ4), utilized in the clinical oncology, [86] the crystal structure of human PTPN2 with inhibitor ABBV-CLS-484 (PDB ID: 7UAD), [87] and the structure of human Thyroid Hormone Receptor Interacting Protein-13 (TRIP13), ATP-bound form (PDB ID: 5VQA), [88] were imported from the protein databank (PDB) for the molecular mechanisms’ prediction. In a comprehensive microarray analysis, TRIP13 was identified as a critical gene implicated in the molecular mechanisms underlying the initiation and progression of melanoma. [89] Elevated TRIP13 expression was observed in malignant tissues, and higher levels were correlated with poorer prognoses in melanoma patients. Mechanistically, TRIP13 interacts with filamin-A (FLNA), triggering the activation of the PI3K/AKT pathway and leading to the upregulation of epithelial-mesenchymal transition (EMT) associated genes. [90]
The matrix remodeling-associated-7 (MXRA7) protein is also reported to play some roles in skin cancer–melanoma pathophysiology and progression. MXRA7 is thought to be involved in extracellular matrix (ECM) remodeling and cellular processes such as adhesion, migration, and signaling. The actual association of MXRA7 in melanoma tumor progression and metastasis has not been fully validated. [46] A glide from the Schrodinger suite was used for molecular docking. [91] Metformin was observed to interact with major residues of the predicted proteins, including SER-187, SER-138 (5VQA), SER-187, SER-138 (5OQ4), and ASP-50 (7UAD), respectively. These interactions were very comparable to the co-crystallized inhibitors of all three proteins used for the target prediction analysis (Figure 7). Thus, further comprehensive studies are essential to elucidate the potential therapeutic targets for metformin in the management of skin cancer (melanoma). The actual 2D interactions of metformin and the protein residues are presented in the Supporting Information.
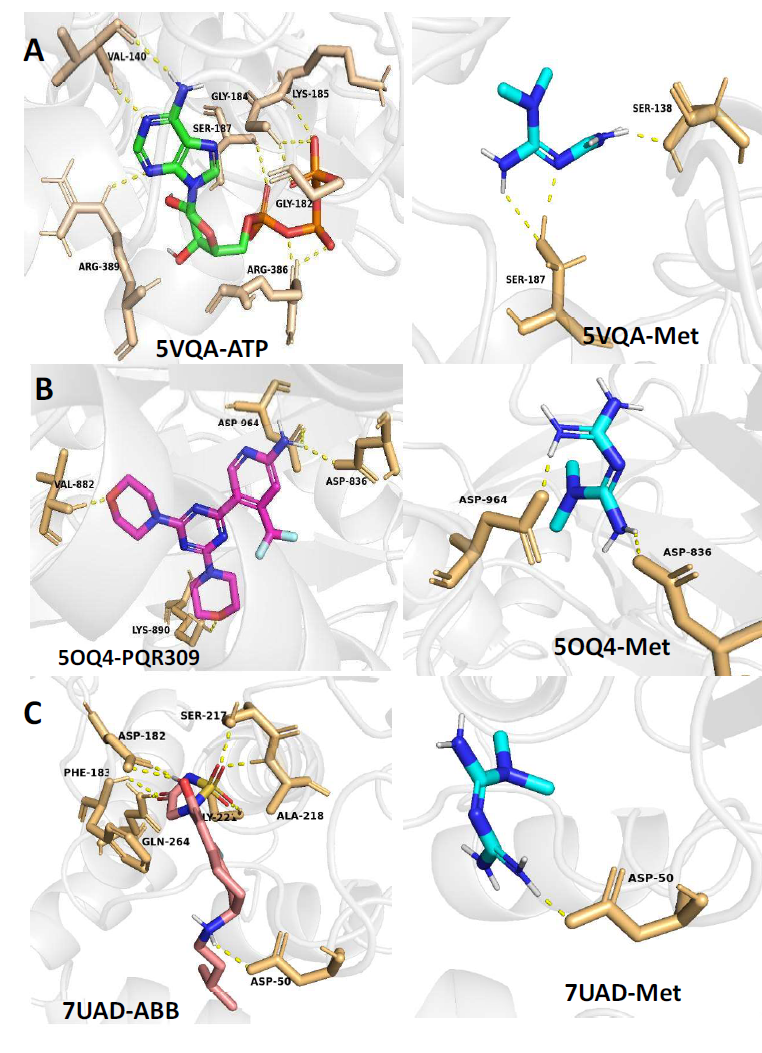
Figure 7: 3D protein-ligand interactions of metformin and standard molecules with 5VQA (A), 5OQ4 (B), and 7UAD (C).
Tseng (2018) examined the link between metformin use and the risk of skin cancer. Using Taiwan’s National Health Research Institute health database, the study retrospectively enrolled 16,237 matched pairs of ever-users and never-users of metformin with new-onset type-2 diabetes diagnosed between 1999 and 2005, following them until December 31, 2011. The incidence of skin cancer was 45.59 and 83.90 per 100,000 person-years among ever-users and never-users, respectively. The results indicate a reduced incidence of skin cancer, including MSC and NMSC, associated with metformin use in a dose-dependent manner among patients with type-2 Diabetes Mellitus. [92] In another retrospective, population-based case-control study, the association between metformin use and keratinocyte carcinoma was investigated among 6,880 patients diagnosed with first-time BCC, SCC, or invasive SCC between 2003 and 2017, alongside 69,620 controls. The study found that metformin use was linked to a lower risk of developing BCC, even at low doses, suggesting its potential as a chemoprotective agent for high-risk BCC patients, though further studies are needed to confirm these findings. [93]
Prospective randomized clinical trials are currently being conducted to assess the clinical benefits of combining metformin with other anticancer drugs. A Phase I/II clinical trial (NCT01638676) in Louisville, United States, is evaluating the therapeutic effects of metformin combined with vemurafenib, a BRAF inhibitor, in 55 patients. Phase I focuses on determining the safety of FDA-approved vemurafenib (960 mg orally, once daily) alongside metformin (500 mg orally, twice daily for two weeks, then 850 mg orally, twice daily) in patients with unresectable Stage IIIC and Stage IV melanoma. Phase II aims to evaluate the clinical efficacy of this combination therapy. The safety profile of the vemurafenib-metformin regimen will be monitored throughout both phases, with treatment administered in 28-day cycles until disease progression or unacceptable toxicity occurs. [94] Additionally, a clinical trial at the University of Louisville is investigating the combination of dabrafenib, trametinib, and metformin. This Phase I/II trial, initiated in 2014 with 53 participants, has not yet reported results. However, researchers hypothesize that combining a non-toxic, FDA-approved dose of oral metformin with the B-Raf inhibitor dabrafenib and the MEK inhibitor trametinib will result in minimal toxicity while improving clinical outcomes, including objective response rates and survival, in patients with metastatic melanoma. [95] Another Phase 1 study in Pittsburgh is currently examining the efficacy and safety of combining pembrolizumab (KEYTRUDA®), an immunotherapeutic agent, with metformin for the treatment of advanced melanoma. [96]
In melanoma, various preclinical studies have shown that metformin can inhibit melanoma cell proliferation, induce apoptosis, and reduce metastasis. Also, epidemiological studies suggest that diabetic patients taking metformin have a lower incidence of melanoma compared to those on other antidiabetic medications, while in NMSCs, Metformin has demonstrated protective effects against BCC and SCC, the two most common types of NMSCs. [97-99] Reports postulate that metformin may reduce UV-induced DNA damage and inflammation, key drivers of NMSC development, with the ability to reduce oxidative stress and inflammation, making it a promising candidate for skin cancer prevention, particularly in high-risk populations. [85,100]
Exploring the potential role of metformin in skin cancer management is particularly appealing due to its affordability, relative safety, and involvement in modulating energy metabolism, a growing area of interest in cancer research. [101, 102] Numerous studies have used population databases to evaluate cancer risk in diabetic individuals, comparing those treated with metformin to those who were not. Other research has focused on patients with both diabetes and cancer, investigating whether metformin use for diabetes management influences cancer outcomes. Most of these studies are retrospective, with metformin use not being randomized, except in rare cases where it was randomly assigned within diabetes treatment trials. Consequently, interpreting these findings is more complex than it may initially appear. [103] In general, retrospective studies should be seen as hypothesis-generating rather than conclusive. They identify promising avenues and underscore the need for further population-based, translational, and laboratory research. The role of nonrandomized studies examining metformin's impact on cancer burden in diabetics in shaping the rationale for clinical trials in nondiabetics remains debatable. However, data on cancer incidence from the Diabetes Prevention Trial provide additional insights, [104] and other cohorts with randomized long-term metformin exposure will be particularly valuable in this context. It is vital to emphasize that incorporating well-designed companion studies with tissue and serum pharmacodynamic markers, along with drug-level assessments, can significantly enhance these trials. Such integration offers deeper insights beyond simply determining whether the drug is active in preventing or treating skin cancer. If the trials show positive activity, these data can guide future research by identifying responsive subpopulations or suggesting effective drug combinations. On the other hand, if the trials produce negative results, companion studies will help interpret the findings, whether by uncovering technical issues that could be addressed in follow-up studies (e.g., selecting a biguanide with improved pharmacokinetics if drug accumulation in tumors is inadequate) or by providing evidence that the drug lacks benefit even under optimal conditions, thereby supporting the decision to halt its development for that specific indication. [105]
Therefore, the research on metformin’s potential in skin cancer prevention and treatment must focus on several key areas: refining retrospective data interpretation to minimize bias, elucidating the molecular mechanisms behind the effects of Metformin on the mitochondria, determining the extent of metformin’s indirect and direct anticancer actions, optimizing pharmacokinetics to enhance drug delivery to target tissues, exploring rational drug combinations (e.g., with chemotherapy, glycolysis inhibitors, or PI3K inhibitors), identifying predictive biomarkers for patient selection, and prioritizing clinical trials to assess its efficacy in cancer prevention and treatment. While metformin shows promise, further investigation is needed to define optimal dosing, combinations, and target populations for its use in oncology. [105, 106] The ideal dosage of metformin for anticancer effects is still uncertain, as most studies have used doses higher than those usually prescribed for diabetes. The potential benefits of metformin may differ depending on individual factors like metabolic condition, tumor type, and genetic makeup. While it is typically well-tolerated, metformin can lead to gastrointestinal side effects and, in rare cases, lactic acidosis, particularly in patients with kidney problems.
The exploration of metformin as a novel therapeutic agent in skin cancer pharmacotherapy presents a promising frontier in oncology. [107] However, to fully harness its potential, several key research and clinical application areas must be addressed. Translational and clinical research expansion is essential to establish metformin’s efficacy and safety in skin cancer treatment. Despite compelling preclinical and epidemiological evidence supporting its anti-tumor properties, large-scale, randomized clinical trials are needed. Personalized medicine and biomarker development will play a key role in optimizing metformin’s use in oncology. Identifying biomarkers that can predict patient response is essential in determining which individuals are most likely to benefit from treatment. For example, research has suggested that metformin exposure is associated with a decreased risk of non-melanoma skin cancers, particularly basal cell carcinoma, across various sex and ethnicity groups. [108] Combination therapies and synergistic approaches have the potential to enhance metformin’s therapeutic efficacy and overcome treatment resistance in skin cancer. Given metformin’s role in altering tumor metabolism, its synergy with glycolysis inhibitors should also be examined as a potential strategy for enhancing cancer therapy. Understanding mechanistic pathways beyond AMPK is another critical area for future research. Although AMPK activation is a central pathway in metformin’s anti-cancer effects, other underlying mechanisms remain unexplored. Examining the interaction between metformin and the tumor microenvironment, including its influence on angiogenesis and immune evasion, could further uncover its therapeutic potential in treating skin cancer. Moreover, metformin's ability to lower insulin-like growth factor-1 (IGF-1) levels and activate AMPK and energy conservation further highlights its broad anticancer properties. Metformin represents a promising, low-cost, and widely available drug with potential applications in skin cancer prevention and treatment. Future research should focus on identifying biomarkers to predict response to metformin, conducting large-scale randomized controlled trials to establish its efficacy, and exploring combination therapies to enhance its anticancer effects. Overall, metformin is a fascinating example of drug repurposing in oncology. [109, 110] Its potential role in skin cancer pharmacotherapy highlights the importance of further research to fully understand its mechanisms and therapeutic potential.
ACKNOWLEDGMENT
The authors appreciate all staff of the Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa State, and members of the Pharmaseries Healthcare Foundation, especially the Pharmaseries Mentorship Group (PMG).
AUTHORS’ CONTRIBUTION
All authors have significantly contributed to the work, whether by conducting literature searches, drafting, revising, or critically reviewing the article. They have given their final approval of the version to be published, have agreed with the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
LIST OF ABBREVIATIONS
ACC2 acetyl-CoA carboxylase-2
AMPK AMP-activated protein kinase
ARID2 AT-rich interaction domain 2
BCC basal cell carcinoma
BRAF B-Raf proto-oncogene
CDKN2A cyclin-dependent kinase inhibitor 2A
COX-2 cyclooxygenase-2
GLUT-1 glucose transporter 1
HFFs human foreskin fibroblasts
HIF-1 hypoxia-inducible factor-1
IGF-1 insulin growth factor-1
IL-2 Interleukin-2
IRS-1 insulin receptor substrates 1
KIT KIT proto-oncogene receptor tyrosine kinase
LKB1 Liver Kinase B1
MC1R melanocortin 1 receptor
MSC melanoma skin cancer
MSH melanocyte-stimulating hormone
MXRA7 matrix remodeling associated-7
NF1 neurofibromin 1
NMSCs non-melanoma skin cancers
OCTs Organic Cation Transporters
PMAT plasma membrane monoamine transporter
PTEN phosphatase and tensin homolog
ROS reactive oxygen species
SCC squamous cell carcinoma
T2DM type 2 diabetes mellitus
TERT telomerase reverse transcriptase
TP53 tumor protein p53
TRIP13 Thyroid Hormone Receptor Interacting Protein-13
T-VEC Talimogene laherparepvec
UVR ultraviolet radiation
XPC xeroderma pigmentosum C
References
- Hofmann E, Schwarz A, Fink J, Kamolz LP, & Kotzbeck P. Modeling the complexity of human skin in vitro. Biomedicines. 2023; 11(3): 794.
- Carter E. Identifying types of skin cancer, risk factors, and effective treatments. International Journal of Advanced Engineering Technologies and Innovations. 2024; 10(2): 79-98.
- Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile, et al. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Medicine. 2021; 9: 20503121211034366.
- Iragorri N, de Oliveira C, Fitzgerald N, & Essue B. The out-of-pocket cost burden of cancer care—a systematic literature review. Current Oncology. 2021; 28(2): 1216-1248.
- Lv Z, & Guo Y. Metformin and Its Benefits for Various Diseases. Frontiers in endocrinology. 2020; 11, 191.
- Roky AH, Islam MM, Ahasan AMF, Mostaq MS, Mahmud MZ, Amin MN, et al. Overview of skin cancer types and prevalence rates across continents. Cancer Pathogenesis and Therapy. 2024; 2: E01-E36.
- Urban K, Mehrmal S, Uppal P, Giesey RL, & Delost GR. The global burden of skin cancer: A longitudinal analysis from the Global Burden of Disease Study, 1990–2017. JAAD international. 2021; 2: 98-108.
- Dode E, Bunu SJ, & Ikoi AI. Comparative in-vitro bioequivalence analysis of metformin hydrochloride tablet formulations available in Yenagoa Metropolis, Bayelsa State, Nigeria. J. Chem. Res. Adv. 2023; 04(01): 21-26.
- Patel JJ, & Mundi MS. Metformin for Type 2 Diabetes. JAMA. 2019; 322(13): 1312.
- Bailey CJ, & Day C. Metformin: its botanical background. Practical diabetes international. 2004; 21(3): 115-117.
- Bailey CJ, & Day C. Traditional plant medicines as treatments for diabetes. Diabetes care. 1989; 12(8): 553-564.
- Bailey CJ, & Turner RC. Metformin. New England Journal of Medicine. 1996; 334(9): 574-579.
- Bailey CJ. Metformin: historical overview. Diabetologia. 2017; 60(9):1566-1576.
- World BioChemicals Industries Ltd. (n.d.). Metformin HCl. Available at: https://www.wbcil.com/organic-molecules/metformin-hcl/. Accessed April 05, 2025.
- Werner EA, Bell J. CCXIV, The preparation of methylguanidine, and ββ-dimethylguanidine by the interaction of dicyanodiamide, and methylammonium and dimethylammonium chlorides, respectively. Journal of the Chemical Society, Transactions. 1922; 121: 1790-1794.
- Pasik C. Diabetes and the biguanides: the mystery of each. Glucophage: serving diabetology. 1997; 40: 79.
- Rathke B. Ueber biguanid. Berichte der deutschen chemischen Gesellschaft. 1879 12(1): 776-784.
- Claro AE, Palanza C, Mazza M, Schuenemann GEU, Rigoni M, Pontecorvi A, et al. Historical use of medicinal plants and future potential from phytotherapy to phytochemicals. Annali di Botanica. 2024; 14(1).
- Chen KK, Anderson RC. The toxicity and general pharmacology of N1-p-chlorophenyl-N5-isopropyl biguanide. The Journal of Pharmacology and Experimental Therapeutics. 1947; 91(2): 157-160.
- Curd FHS, Davey DG, Rose FL. Studies on synthetic antimalarial drugs; some biguanide derivatives as new types of antimalarial substances with both therapeutic and causal prophylactic activity. Annals of Tropical Medicine and Parasitology. 1945; 39: 208-216.
- Sterne J. Treatment of diabetes mellitus with N, N-dimethylguanylguanidine (LA. 6023, Glucophage). Therapie. 1959; 14: 625-630.
- McKendry JBR, Kuwayti K, & Rado PP. Clinical experience with DBI (phenformin) in the management of diabetes. Canadian Medical Association Journal. 1959; 80(10): 773.
- Ungar G, Freedman L, & Shapiro SL. Pharmacological studies of a new oral hypoglycemic drug. Proceedings of the Society for Experimental Biology and Medicine. 1959; 95(1): 190-192.
- Lalau J. D. Lactic acidosis induced by metformin: incidence, management and prevention. Drug safety. 2010; 33(9): 727-740.
- Luft D, Schmülling RM, & Eggstein M. Lactic acidosis in biguanide-treated diabetics: a review of 330 cases. Diabetologia. 1978; 14: 75-87.
- Bailey CJ, & Nattrass M. 11 Treatment—metformin. Bailliere's clinical endocrinology and metabolism. 1988; 2(2): 455-476.
- Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discovery. 2012; 2(9): 778-790.
- Zhao H, Swanson KD, Zheng B. Therapeutic Repurposing of Biguanides in Cancer. Trends in Cancer. 2021; 7(8): 714-730.
- Bailey CJ, Campbell IW, Chan JCN, Davidson JA, Howlett HCS, Ritz P. (Eds.). Metformin – The gold standard: a scientific handbook. Wiley, 2007. Retrieved February 28, 2025, from https://research.aston.ac.uk/en/publications/metformin-the-gold-standard-a-scientific-handbook.
- DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. The New England Journal of Medicine. 1995; 333(9): 541-549.
- Kasznicki J, Sliwinska A, & Drzewoski J. Metformin in cancer prevention and therapy. Annals of translational medicine. 2014; 2(6): 57.
- Zhang Y, Zhou F, Guan J, Zhou L, & Chen B. Action mechanism of metformin and its application in hematological malignancy treatments: a review. Biomolecules. 2023; 13(2): 250.
- Vial G, Detaille D, & Guigas B. Role of mitochondria in the mechanism (s) of action of metformin. Frontiers in endocrinology. 2019; 10: 294.
- Khilnani G. Metformin: A Reflection on My Journey as an Antidiabetic Drug. GAIMS Journal of Medical Sciences. 2023; 3(2): 34-40.
- Gordon R. (2013). Skin cancer: an overview of epidemiology and risk factors. In Seminars in Oncology Nursing. WB Saunders. 2013; 29(3): 160-169.
- Khan NH, Mir M, Qian L, Baloch M, Khan MFA, Ngowi EE, et al. Skin cancer biology and barriers to treatment: Recent applications of polymeric micro/nanostructures. Journal of Advanced Research. 2022; 36: 223-247.
- Šerman N, Vranić S, Glibo M, Šerman L, & Mokos ZB. Genetic risk factors in melanoma etiopathogenesis and the role of genetic counseling: A concise review. Bosnian journal of basic medical sciences. 2022; 22(5): 673.
- Wu S, Cho E, Li, WQ, Weinstock MA, Han J, & Qureshi AA. History of severe sunburn and risk of skin cancer among women and men in 2 prospective cohort studies. American journal of epidemiology. 2016; 183(9): 824-833.
- Ciążyńska M, Kamińska-Winciorek G, Lange D, Lewandowski B, Reich A, Sławińska M, et al. The incidence and clinical analysis of non-melanoma skin cancer. Scientific reports. 2021; 11(1): 4337.
- Griffin LL, Ali FR, & Lear JT. Non-melanoma skin cancer. Clinical medicine. 2016; 16(1): 62-65.
- Kasumagic-Halilovic E, Hasic M, & Ovcina-Kurtovic N. A clinical study of basal cell carcinoma. Medical archives. 2019; 73(6): 394.
- Kallini JR, Hamed N, & Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. International journal of dermatology. 2015; 54(2): 130-140.
- Leonardi GC, Falzone L, Salemi R, et al. Cutaneous melanoma: From pathogenesis to therapy (Review). International Journal of Oncology. 2018 52: 1071-1080.
- Liu-Smith F, Jia J, & Zheng Y. UV-induced molecular signaling differences in melanoma and non-melanoma skin cancer. Ultraviolet light in human health, diseases, and the environment. 2017; 27-40.
- Bunu SJ, Cai H, Wu L, Zhang H, Zhou Z, Xu Z, et al. TRIP13 - a potential drug target in cancer pharmacotherapy. Bioorganic chemistry. 2024; 151: 107650.
- Wang Y. Matrix Remodeling Associated 7 (MXRA7): A Long-lost Member of the Non-kin MXRA Family. Chinese Journal of Biochemistry and Molecular Biology. 2020; 36(7): 725-733
- Jaune E, & Rocchi S. Metformin: Focus on melanoma. Frontiers in endocrinology. 2018; 9: 472.
- Tseng HW, Shiue YL, Tsai KW, Huang WC, Tang PL, & Lam HC. Risk of skin cancer in patients with diabetes mellitus: a nationwide retrospective cohort study in Taiwan. Medicine. 2016; 95(26): e4070.
- Koury J, Lucero M, Cato C, Chang L, Geiger J, Henry, D, et al. Immunotherapies: Exploiting the Immune System for Cancer Treatment. Journal of Immunology Research. 2018; 2018: 9585614.
- Kumar AR, Devan AR, Nair B, Vinod BS, & Nath LR. Harnessing the immune system against cancer: current immunotherapy approaches and therapeutic targets. Molecular biology reports. 2021; 48(12): 8075–8095.
- Czarnecka AM, Bartnik E, Fiedorowicz M, & Rutkowski P. Targeted Therapy in Melanoma and Mechanisms of Resistance. International journal of molecular sciences. 2020; 21(13): 4576.
- Proietti I, Skroza N, Michelini S, Mambrin A, Balduzzi V, Bernardini N, et al. BRAF Inhibitors: Molecular Targeting and Immunomodulatory Actions. Cancers. 2020; 12(7): 1823.
- Serrone L, Zeuli M, Sega FM, & Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. Journal of experimental & clinical cancer research: CR. 2000; 19(1): 21–34.
- Rao RD, Holtan SG, Ingle JN, Croghan GA, Kottschade LA, Creagan ET, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006; 106(2): 375–382.
- Kaur R, Bhardwaj A, & Gupta S. Cancer treatment therapies: traditional to modern approaches to combat cancers. Molecular biology reports. 2023; 50(11): 9663–9676.
- Mehta A, Motavaf M, Nebo I, Luyten S, Osei-Opare KD, & Gru AA. Advancements in Melanoma Treatment: A Review of PD-1 Inhibitors, T-VEC, mRNA Vaccines, and Tumor-Infiltrating Lymphocyte Therapy in an Evolving Landscape of Immunotherapy. Journal of Clinical Medicine. 2025; 14(4): 1200.
- Miller RA, & Birnbaum MJ. An energetic tale of AMPK-independent effects of metformin. The Journal of Clinical Investigation. 2010; 120(7): 2267-2270.
- Ke R, Xu Q, Li C, Luo L, & Huang D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biology International. 2018; 42(4): 384-392.
- Gonga L, Goswamic S, Giacominic KM, Altmana RB, & Kleina TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012; 22(11): 820-827.
- Lewis A, Williams K, & Oroszi T. Metformin—Pharmacokinetic and Pharmacodynamics Journey Through the Body. Pharmacology & Pharmacy. 2024;15(12): 466-477.
- San KIH. The use of metformin in patients with renal impairment; Doctoral dissertation, University of Otago, 2020.
- Yasin MT. A review on metformin for the treatment of type II Diabetes Mellitus; Doctoral dissertation, Brac University, 2024.
- Saleh J, Yusuph H, & Aji B. Combination of Metformin and Thiazolidinediones in Type 2 Diabetes Mellitus- A Review. Nigerian Hospital Practice. 2009; 3: 3-4.
- Gong L, Goswami S, Giacomini KM, Altman RB, & Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenetics and Genomics. 2012; 22(11): 820–827.
- Bristol-Myers S. GLUCOPHAGE- metformin hydrochloride tablet, film-coated GLUCOPHAGE XR- metformin hydrochloride tablet, extended-release, patient information leaflet, 2019, https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=453104.
- Heller JB. Metformin overdose in dogs and cats. Veterinary Medicine-Bonner Springs then Edwardsville. 2007; 102(4): 231.
- Hougen I, Whitlock RH, Komenda P, Rigatto C, Clemens KK, & Tangri N. Safety of add-on sulfonylurea therapy in patients with type 2 diabetes using metformin: a population-based real-world study. BMJ Open Diabetes Research & Care. 2021; 9(2): e002352.
- Sheleme T. Clinical Pharmacokinetics of Metformin. In www.intechopen.com. IntechOpen, 2021. https://www.intechopen.com/chapters/77996.
- Lei Y, Yi Y, Liu Y, Liu X, Keller ET, Qian C, et al. Metformin targets multiple signaling pathways in cancer. Chinese Journal of Cancer. 2017; 36(1): 17.
- Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife. 2014: 3: e02242.
- Hur KY, & Lee MS. New mechanisms of metformin action: Focusing on mitochondria and the gut. Journal of diabetes investigation. 2015; 6(6): 600-609.
- Meng S, Cao J, He Q, Xiong L, Chang E, Radovick S, et al. Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. The Journal of Biological Chemistry. 2015; 290(6): 3793–3802.
- Kim I, & He YY. Targeting the AMP-Activated Protein Kinase for Cancer Prevention and Therapy. Frontiers in Oncology. 2013; 3: 175.
- Gallagher EJ, & LeRoith D. Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Annals of the New York Academy of Sciences. 2011; 1243(1): 54-68.
- Ding XZ, Fehsenfeld DM, Murphy LO, Permert J, & Adrian TE. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase, and enhancing GLUT-1 expression. Pancreas. 2000; 21(3): 310-320.
- Draznin B. Mechanism of the mitogenic influence of hyperinsulinemia. Diabetology & metabolic syndrome. 2011; 3: 1-3.
- Morales DR, & Morris AD. Metformin in cancer treatment and prevention. Annual review of medicine. 2015; 66(1): 17-29.
- Sachdev D, & Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Molecular cancer therapeutics. 2007; 6(1): 1-12.
- Algire C, Amrein L, Bazile M, David S, Zakikhani M, & Pollak M. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene. 2011 30(10): 1174-1182.
- Gong J, Kelekar G, Shen J, Shen J, Kaur S, & Mita M. The expanding role of metformin in cancer: an update on antitumor mechanisms and clinical development. Targeted oncology. 2016; 11: 447-467.
- Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, et al. IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. Journal of Surgical Research. 2010; 160(1): 90-101.
- Kourelis TV, & Siegel RD. Metformin and cancer: new applications for an old drug. Medical oncology. 2012; 29: 1314-1327.
- Lewis D, Travers J, Somani AK & Spandau DF. The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene. 2010; 29: 1475–1485.
- Salminen A, Kaarniranta K, & Kauppinen A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflammation Research. 2022; 71(7): 817-831.
- Chen Q, Zhang H, Yang Y, Zhang S, Wang J, Zhang D, et al. Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway. International Journal of Molecular Sciences. 2022; 23(13): 6960.
- Beaufils F, Cmiljanovic N, Cmiljanovic V, Bohnacker T, Melone A, Marone R, et al. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (PQR309), a Potent, Brain-Penetrant, Orally Bioavailable, Pan-Class I PI3K/mTOR Inhibitor as Clinical Candidate in Oncology. Journal of Medicinal Chemistry. 2017; 60(17): 7524–7538.
- Baumgartner CK, Ebrahimi-Nik H, Iracheta-Vellve A, Hamel KM, Olander KE, Davis TGR, et al. The PTPN2/PTPN1 inhibitor ABBV-CLS-484 unleashes potent anti-tumour immunity. Nature. 2023; 622(7984): 850–862.
- Ye Q, Kim DH, Dereli I, Rosenberg SC, Hagemann G, Herzog F, et al. The AAA+ ATPase TRIP13 remodels HORMA domains through N-terminal engagement and unfolding. The EMBO Journal. 2017; 36(16): 2419–2434.
- Ma J, Cai X, Kang L, Chen S, & Liu H. Identification of novel biomarkers and candidate small-molecule drugs in cutaneous melanoma by comprehensive gene microarray analysis. Journal of Cancer. 2021; 12(5): 1307–1317.
- Lu W, Mengxuan Z, Ming R, Zixu G, Yong Z, Simin Z, et al. TRIP13/FLNA Complex Promotes Tumor Progression and Is Associated with Unfavorable Outcomes in Melanoma. Journal of Oncology. 2022: 1419179.
- Schrödinger. Maestro Programme, Schrödinger. LLC, New York, NY. S, 2020.
- Tseng CH. Metformin is associated with decreased skin cancer risk in Taiwanese patients with type 2 diabetes. Journal of the American Academy of Dermatology. 2018; 78(4): 694-700.
- Adalsteinsson JA, Muzumdar S, Waldman R, Wu R, Ratner D, Feng H, et al. Metformin is associated with decreased risk of basal cell carcinoma: A whole-population case-control study from Iceland. Journal of the American Academy of Dermatology. 2021; 85(1): 56-61.
- ClinicalTrials.gov. (n.d.). A Phase I/II trial of vemurafenib and metformin to melanoma patients - Full-text view. U.S. National Library of Medicine. Retrieved from https://clinicaltrials.gov/ct2/show/NCT01638676
- ClinicalTrials.gov. (n.d.). Efficacy and safety of metformin in patients with type 1 diabetes (NCT02143050). U.S. National Library of Medicine. Retrieved 27th February 2025, from https://clinicaltrials.gov/study/NCT02143050.
- ClinicalTrials.gov. (n.d.). Study of Metformin in Patients with Kidney Disease (NCT03311308). U.S. National Library of Medicine. Retrieved 27th February 2025, from https://clinicaltrials.gov/study/NCT03311308.
- Saraei P, Asadi I, Kakar MA, & Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer management and research. 2019; 11: 3295–3313.
- Suwei D, Yanbin X, Jianqiang W, Xiang M, Zhuohui P, Jianping K, et al. Metformin inhibits melanoma cell metastasis by suppressing the miR-5100/SPINK5/STAT3 axis. Cellular & molecular biology letters. 2022 27(1): 48.
- Tomic T, Botton T, Cerezo M, Robert G, Luciano F, Puissant A, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell death & disease. 2011; 2(9): e199.
- Souza-Neto FP, Marinello PC, Melo GP, Ramalho LZN, Cela EM, Campo VE, et al. Metformin inhibits the inflammatory and oxidative stress response induced by skin UVB irradiation and provides 4-hydroxy-2-nonenal and nitrotyrosine formation and p53 protein activation. Journal of Dermatological Science. 2020; 100(2): 152–155.
- Vander HMG. Targeting cancer metabolism: a therapeutic window opens. Nature reviews Drug discovery. 2011; 10(9): 671-684.
- Ward PS, & Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer cell. 2012; 21(3): 297-308.
- Carstensen B, Witte DR, & Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia. 2012; 55: 948-958.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, & Walker. Reduction in the incidence 3 of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(4): 393–403.
- Pollak M. Metformin and Other Biguanides in Oncology: Advancing the Research Agenda. Cancer Prevention Research (Philadelphia, Pa.). 2010; 3(9): 1060-1065.
- Kumbhar P, Kole K, Yadav T, Bhavar A, Waghmare P, Bhokare R, et al. Drug repurposing: An emerging strategy in alleviating skin cancer. European Journal of Pharmacology. 2022; 926: 175031.
- Feng H, Shang S, Chen K, Sun X, & Yue X. Impact of metformin on melanoma: a meta-analysis and systematic review. Frontiers in Oncology. 2024; 14: 1399693.
- Haq Z, Mirza FN, Abdi P, Diaz MJ, & Libby TJ. Metformin Use and Risk of Non-Melanoma Skin Cancer: A Propensity-Matched Case-Control Study. Journal of Drugs in Dermatology. 2024; 23(12): 1089–1095.
- Weir SJ, DeGennaro LJ, & Austin CP. Repurposing approved and abandoned drugs for the treatment and prevention of cancer through public-private partnerships. Cancer Research. 2012; 72(5): 1055-1058.
- Xia Y, Sun M, Huang H, & Jin WL. Drug repurposing for cancer therapy. Signal transduction and targeted therapy. 2024; 9(1): 92.
