Full HTML
Venous Thromboembolism During Pregnancy and Postpartum: An Updated Review
Elmukhtar Habas1, Eshrak Habas2, Amnna Rayani3, Ala Habas2, Khalid Alarbi4, Jamal Alfitori5, Aml Habas2, Kalifa Farfar6, Almehdi Errayes5, Abdel-Naser Elzouki5.
Author Affiliation
1 Professor/Senior Consultant, Department of Medicine, Hamad General Hospital, Qatar University, Doha-Qatar, Open Libyan University, Libya
2 Resident, Department of Medicine, Tripoli Central Hospital, University of Tripoli, Tripoli-Libya
3 Professor/Senior Consultant, Tripoli Children Hospital, Open Libyan University, Tripoli-Libya
4 Specialist, Department of Medicine, Hamad General Hospital, Doha-Qatar
5 Senior Consultant, Department of Medicine, Hamad General Hospital, Doha-Qata
6 Consultant, Department of Medicine, Alwakra General Hospital, Alwakra-Qatar.
Abstract
Venous thromboembolism (VTE) is a serious medical condition that has a high clinical burden on both the mother and fetus, despite having a low incidence during pregnancy and the postpartum period. VTE is a leading cause of death among pregnant women and new mothers worldwide, and its severity cannot be underestimated. The development of VTE is influenced by a combination of genetic and environmental factors, as well as acquired conditions. Pregnancy-related changes, such as increased levels of coagulation factors and diminished fibrinolysis, can increase the risk of VTE. Additionally, older mothers, those who are obese, multiparous, or have undergone cesarean delivery, are at a higher risk of developing VTE. Owing to the lack of standardized guidelines and reliable data on VTE prevention, diagnosis, and treatment, authorities have developed risk scores that allow for a personalized assessment of the risk of thrombosis during pregnancy and postpartum, enabling a tailored approach to prevent thrombosis. Managing VTE during pregnancy poses significant challenges because the benefits and risks of anticoagulant treatment for both the mother and fetus must be carefully balanced. An interdisciplinary approach that includes obstetricians, neonatologists, physicians, and hematologists is essential to achieving optimal outcomes. This review explored the VTE updates in pathogenesis, presentation, complications, treatment options, and research gaps with proposal strategies to improve VTE outcomes and suggest further research.
DOI: 10.63475/yjm.v4i1.0030
Keywords: Venous thromboembolism (VTE), venous embolism, pregnancy, anticoagulation, Deep vein thrombosis (DVT).
Pages: 79-94
View: 5
Download: 20
DOI URL: https://doi.org/10.63475/yjm.v4i1.0030
Publish Date: 22-05-2025
Full Text
Pregnancy increases venous thromboembolism (VTE) risk by 2 – 5-fold. VTE is a notable illness during pregnancy, occurring in around 1/1000 pregnancies (reported incidence: 0.025% to 0.1%). [1,2] VTE during pregnancy had a significant negative impact on physical and professional life, mental health, and future pregnancy plans. [3]
In UK VTE mortality is 1.5/100,000 pregnancies. [4] Despite national treatment and preventive guidelines, the 2018 MBRRACE study indicated that VTE mortality has declined since 2009. [5]
Between 2019 and 2021, a total of 241 women died during or within six weeks after giving birth out of a population of 2,066,997 women in the UK. The maternal fatality rate was 11.7/100,000 women, including deaths that occurred during pregnancy up to 6 weeks after delivery. [5] The primary factors that contributed to mortality were COVID-19, cardiovascular disorders, and thrombosis (14%). [5] Most VTE incidents (20 - 50%) occur in the initial 6 weeks following birth. About half of prenatal incidents occur in the first or second trimester. [4]. Although there have been improvements in the methods used to diagnose and treat VTE, VTE remains a significant cause of death among pregnant females in industrialized nations. VTE accounts for around 10% of maternal fatalities in the US, with pulmonary embolism (PE) as the predominant underlying cause of death. This percentage was higher than the deaths caused by other pregnancy-related problems, such as infection, cardiovascular illness, cardiomyopathies, amniotic fluid embolism, anesthesia complications, hemorrhage, and gestational hypertension (HTN). [6] Utilizing the Pregnancy Mortality Surveillance System (PMSS), one can compute the pregnancy-related mortality ratio, which provides an approximation of the fatalities attributed to pregnancy/100,000 live births. According to PMSS, a pregnancy-associated death is defined as the woman dying during the pregnancy or within a year of postpartum, caused by any factor that is connected to or made worse by the pregnancy. [7] After the implementation of the PMSS, the incidence of reported pregnancy-related fatalities in the US rose from 7.2 deaths/100,000 in 1987 to 17.6 deaths/100,000 in 2019. The causes behind the overall rise in pregnancy-related mortality remain unclear. The implementation of computerized data links between birth and death records and child death records, modifications in the coding of causes of fatality, and the inclusion of a pregnant checkbox in death records have enhanced the identification of pregnancy-related fatalities. Nevertheless, inaccuracies in the documentation of pregnant status on fatality records have been documented, which might result in an overestimation of the incidence of fatalities connected to pregnancy. [8] However, it is vital to acknowledge that if VTE is identified only by a clinical assessment, there is a possibility of overestimating the occurrence of the illness.
VTE occurrence varies across various demographic groups and is influenced by distinct risk factors. For instance, those who have already had thrombophilia or VTE, those who are undergoing assisted reproductive therapies, and those with chronic illnesses like diabetes or hypertension (HTN) have a greater likelihood of developing VTE. Moreover, there have been documented differences in the occurrence of VTE during pregnancy based on race and ethnicity, with African Americans having a greater susceptibility compared to White-Caucasian women.[9]
Understanding the risk factors and pathogenesis of VTE during pregnancy is vital due to its high occurrence, the resulting health problems and deaths, and the hurdles in diagnosing it. This information may educate the patients and practitioners and develop evidence-based strategies to prevent and treat VTE during and after pregnancy. This review article offers a thorough understanding of pregnancy-induced VTE, with a focus on the difficulties of diagnosing, treating, and pathogenesis, and the latest practical approaches to tackle these obstacles of pregnancy-associated VTE. We intend to make this review a helpful resource for healthcare practitioners and academics interested in improving VTE outcomes for pregnant females by integrating evidence-based information and highlighting areas of lacking understanding. Different texts, keywords, and phrases DVT in pregnancy, DVT Complications in pregnancy, DVT risk in pregnancy, factors affecting the pulmonary embolism occurrence in pregnancy, DVT in childbearing age, anticoagulants therapy of VTE during pregnancy, and others were used to search for original and review article in PubMed, EMBASE, Google Scholar, and Google, which are published between Jan 2000-Dec 2024.
The absolute VTE incidence in pregnancy is 1 - 2 cases/1,000 pregnancies and one fatality/100,000 deliveries. [10,11] VTE risk was 5-fold higher during pregnancy and 60-fold higher in the first 3 months following birth than in nonpregnant women. [10,12] A meta-analysis review of 20 studies revealed that the collective overall incidence of pregnancy-linked VTE was 1.2/1000 deliveries. [3] VTE rates in Europe and the US range from approximately 1–2 per 1,000 person-years; [13] however, they vary by age, sex, race, and comorbidities. VTE is estimated at 0.2/1,000 person-years in South Korea [14]. Africa's VTE rate is unknown [15]. Lee et al. stated that Asian population-wide incidence estimates were 15–20% of Western levels but had grown over time. [16] Oceania and South America have less data. [17] The incidence of VTE in Buenos Aires, Argentina, and Perth, Australia, was 0.7 and 0.8 per 1,000 person-years, respectively. [18] In consideration of the prevalence of high parity, obesity, advanced maternal age, frequent cesarean sections, and consanguineous marriages that increase inherited thrombophilia risk, the VTE risk among Saudi Arabian women is deemed to be high. It was reported that they experienced 1.25 cases of VTE per 1000 deliveries. [19] Another study documented a postpartum VTE incidence of 9%, [20] and the awareness about VTE during pregnancy was very low in Saudi Arabia. [21]
An investigation of 395,335 pregnancies compared to control cases at 24 weeks of pregnancy found that the occurrence of VTE was 85 cases/100,000 pregnancies. [22] In another study conducted over 30 years in a community setting, it was discovered that 200 women/100,000 person-years had been diagnosed with VTE [10] According to retrospective research including over 72,000 deliveries, DVT occurrence was demonstrated to be 0.7/1000 births (0.5/1000 occurred during pregnancy, and 0.2/1000 happened after delivery). [23] The research found that the occurrence of pulmonary embolism (PE) was 0.15/1000 (0.07 cases/1000 before delivery and 0.08 cases/1000 after delivery). [23] The data about VTE in the antepartum period shows considerable inconsistencies, with several studies reporting a more extraordinary occurrence during the second and third trimesters. [24,25] On the other hand, several researchers have shown that VTE risk is the same across all pregnancy trimesters. [1,10,26] It is agreed that VTE risk is most significant during the postpartum period, particularly during the first 6 weeks. [12]
VTE is a principal contributor to maternal fatality and morbidity, leading to 13.8% of maternal deaths in civilized nations and 3.2% globally. [27] The most common underlying cause of death in VTE is PE in women. A meta-analysis revealed that the pooled VTE case fatality rate was 0.68%, and the recurrence rate was 4.27%. [3] Another study reported that the pooled VTE case fatality rate was 0.68%. [3] In Saudi Arabia, a reported mortality rate was 0.025 case/1,000 deliveries. [19]
Virchow's triad consists of hypercoagulability, venous stasis, and vascular endothelium injury pillars, which usually coexist during postpartum and pregnancy, increasing the VTE risk. [28] Gravid uterus enlargement compresses the left common iliac and inferior vena cava (IVC), causing venous stasis. [29,30] Physiological hypercoagulability occurs during pregnancy due to increased clotting factor concentrations, fibrinolysis inhibition, and decreased anticoagulant activity. [31] Moreover, increased circulating growth factors and cytokines during a normal pregnancy may exacerbate endothelium disintegration, producing vascular malfunction and damage. [32] Both surgical delivery and regular labor might result in endothelial damage. [33] Furthermore, increased blood volume and artery diameter induce extreme vessel stress, possibly resulting in vascular injury. [32]. All these changes in the pelvic and lower limb veins increase DVT risk. [34]
DVT during pregnancy is more common in the left leg (85%) and in proximal sites (72% in the iliofemoral veins vs. 9% in nonpregnant women). [35] Post-thrombotic syndrome and embolic consequences are more likely in pregnant women. [36] Half of pregnancy's thrombotic attacks occur throughout the prenatal and postnatal periods. [37] DVTs are 3 times as common as PE in pregnancy. [10] DVT and PE occur in two-thirds of cases during pregnancy and postpartum, respectively. [38] The first three weeks after birth account for 80% of postpartum thromboembolic events. [39] Risk factors for DVT and VTE increase during the 12 weeks after birth. [40]
Previous VTE and thrombophilia are the most significant prenatal and postnatal risk factors. [41] VTE recurrence during pregnancy is 3.5 times higher than in nonpregnant females. The likelihood of recurrence seems to remain consistent throughout pregnancy. [42] Recurrence is higher if the previous VTE was spontaneous, occurred during pregnancy, or was related to hormonal contraception. [43,44] VTE risk increases with thrombophilia type, family history, and risk factors. According to a meta-analysis, the absolute VTE risk surpassed 3% only for females with antithrombin, protein C, and protein S deficits or factor V Leiden homozygosity. [45] DVT and VTE risk in women with an antiphospholipid syndrome without a VTE history is high. [11,46]
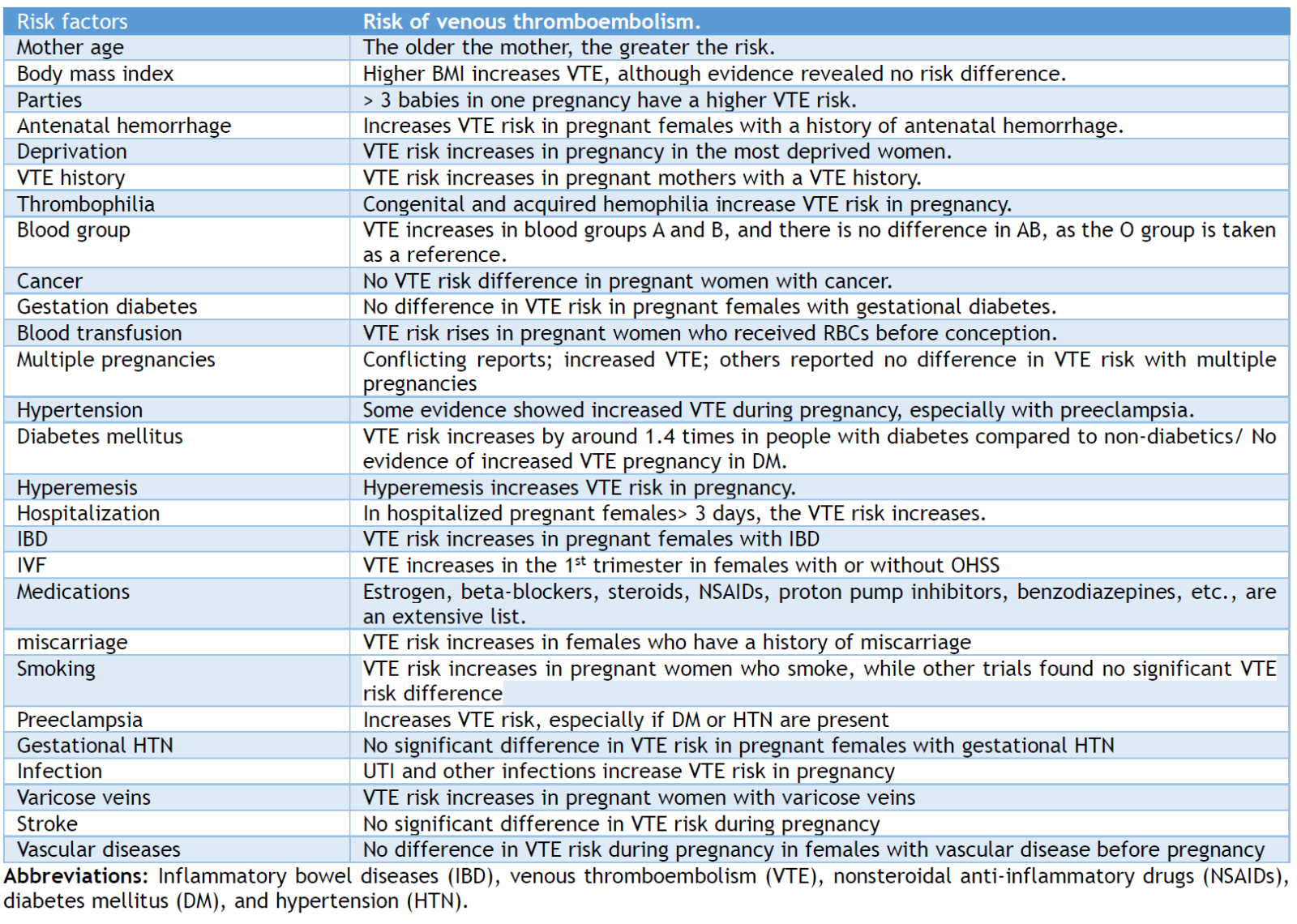
VTE risk is influenced by maternal factors such as body mass index (BMI) ≥ 40 kg/m2 with antepartum immobility and medical comorbidities such as sickle cell disease or diabetes [47,48]. Minor risk factors of VTE include age ≥ 35, BMI ≥ 25 - 40 kg/m2, smoking, parity ≥ 3 pregnancies, in vitro fertilization (IVF), varicose veins, and VTE family history. Hospitalization, surgery, systemic diseases, immobility/long-distance travel, and ovarian hyperstimulation syndrome (OHSS) moderately increase VTE risk during pregnancy. Preeclampsia, hyperemesis, and multiple gestations affect VTE risk. Additionally, packed red blood transfusion, postpartum hemorrhage >1,000 mL, or emergency cesarean section enhances DVT and PE risk. Mild risk factors include stillbirth, preterm delivery (< 37 weeks), protracted labor (> 12 hours), manual placenta removal, and surgical vaginal delivery; all increase PE and DVT. [49] However, the effects of these risk factors vary according to different reports. Over 78% of females had at least one VTE risk factor, and 40% had two or more. [49] A review article reviewed 14 articles to identify VTE-independent risk factors in pregnant women. Risk factors such as pregnancy number, age, antenatal hemorrhage, blood transfusion, blood group, body mass index, deprivation, vaginal and cesarian section delivery, previous DVT, and VTE were analyzed in this meta-analysis review. There were reported to be varied conclusions that were contradictory. These conflicting reports and contradictions could be due to different reasons, such as data collection bias, small number of participants, risk factors rating system, etc. [50] Table 1 summarizes the risk factors that affect the VTE risk in pregnancy and postpartum. The economic cost of DVT and PE during the antenatal/postnatal period was not well studied. However, the short-term higher cost should be balanced against the decreased costs and increased quality of life from improved pregnancy outcomes. The short- and long-term costs and quality of life after VTE during pregnancy have not been widely and extensively studied; hence, extensive multicenter studies are required.
Thrombus formation is primarily caused by the disturbance of Virchow's triad pillars. Virchow's pillars include endothelial damage, hypercoagulability, and venous stasis. During pregnancy and the postpartum period, Virchow's triad equilibrium is disrupted. [1,51] Pregnancy often involves an increase in blood clotting and a decrease in thrombolysis. The alterations in the fibrinolytic and coagulation systems are intended to reduce blood loss during childbirth. However, these changes also, unfortunately, increase the likelihood of developing thromboembolism. During pregnancy, the coagulation system is naturally stimulated because of elevated levels of substances that promote blood clotting and the reduction or deactivation of substances that prevent blood clotting and dissolve blood clots. Factors VII, VIII, X, XIII, von Willebrand factor, and fibrinogen show an increment. [52] Significantly, the levels of factor VII increase dramatically, reaching up to 10 times their original amount. [53]
Additionally, the fibrinogen levels at full term are 200% greater than before pregnancy. [54] Further, there is a notable reduction in physiological anticoagulants, shown by decreased protein S activity and the development of resistance to activated protein C [52]. In addition, the ability to break down blood clots decreases because of increased plasminogen activator inhibitor-1 (PAI-1 and PAI-2) levels. [55]
Pregnancy also affects platelet function and count. Platelet count decreases, but their responsiveness to agonists like adenosine diphosphate (ADP) and thrombin increases, enhancing platelet aggregation. [1,56] This might be partially attributed to alterations in the platelet surface receptors. [57] Placental trophoblastic cells ultimately produce substances that have been demonstrated, in laboratory settings, to have vascular endothelium and prothrombotic effects on platelets. [58] The heightened platelet aggregation also augments the susceptibility to thrombosis, which, coupled with the decrease in fibrinolytic activity, creates a fibrin mesh that is more resilient to breakdown.
Pregnancy is linked to coagulation, platelet function alterations, hormonal levels, hemodynamics, and homeostasis. Pregnancy hemodynamic alterations are marked by an augmentation in intravascular volume and reduced systemic vascular resistance. [59] The rise in progesterone, estrogen, and relaxin levels during pregnancy leads to widespread vasodilation and an increase in venous capacitance. [60] This results in an expansion in vessel diameter and a reduction in blood flow velocity, which can be detected in real time and through DUS. [61] This physiological change promotes blood stagnation in the venous circulation, enhancing blood clot formation. Additionally, mechanical obstructions exacerbate venous pooling because the gravid uterus compresses the iliac veins, impeding venous return. [4,62] Additionally, protracted sitting, standing, or bed rest, which is frequently observed during pregnancy, increases the likelihood of thrombosis. [1,63]
Table 1. The risk factor list predisposes to venous thromboembolism in pregnancy and postpartum
In addition to increasing coagulation factor synthesis and decreasing antithrombin activity, elevated estrogen and progesterone activate the hypoxia-inducible factor-1 pathway, [1,64] activating the platelets and aggregation, promoting hypercoagulability. [1,65] Pregnancy also causes alterations in blood vessel structure, including increased smooth muscle and decreased elastin content. These changes make the vessel wall more susceptible to damage and subsequent thrombus formation. [66] It is increasingly evident that increased vascular compliance, mechanical compression of the major veins in the pelvis, venous stasis, and overall hypercoagulable state, in addition to the delivery-associated endothelial damage, contribute to the formation of a highly thrombogenic milieu, even in normal, uncomplicated pregnancies. [1] Furthermore, the increased vein dilatation and capacitance, blood volume, and venous return decreased venous linear blood flow velocity in the lower limb, resulting in valve incompetence and venous pooling, which promoted thrombus formation. [4]
Pregnancy increases platelet turnover and aggregation, coagulation, and decreases fibrinolysis, making it hypercoagulable. Thrombin, fibrinogen, factor VII, VIII, IX, X, V, and XII rise during pregnancy. [67] Protein C and S (natural anticoagulants) are reduced. [4,68] Platelet count usually stays the same or drops throughout pregnancy. In the first 48 hours following delivery, fibrinolytic activity diminishes, clot stability improves, and platelet activity increases. Additionally, inducible factors and blood stasis due to hormonal and mechanical causes increase thrombosis risk.
Furthermore, acquired or inherited thrombophilia increases the risk of thrombosis during pregnancy and after delivery, resulting in VTE formation. Eight to fifteen percent of Caucasians have thrombophilia, the second most prevalent risk factor for pregnancy-associated VTE. [62] The hereditary form arises from genetic mutations impacting genes in the coagulation cascade. Several inherited thrombophilias, including antithrombin deficiency, factor V Leiden, prothrombin gene mutation, protein C, and protein S abnormalities, have all been linked to an increased VTE risk during pregnancy and after delivery. [69] V Leiden is an inherited thrombophilia with a prevalence of 5% in the Caucasian population. [70] An additional prevalent inherited thrombophilia is protein C abnormality and prothrombin G20210A gene mutation, which affects around 2% of the general population. These mutations cause excess prothrombin, a precursor to thrombin, which subsequently leads to VTE and PE. [70] Hereditary thrombophilia, characterized by deficiencies in antithrombin, protein S, and protein C, is uncommon but strongly linked to VTE development during pregnancy and recurrent miscarriage. [1,71] Protein C and protein S are cofactors in the inactivation of coagulation factors, whereas antithrombin is a naturally occurring anticoagulant that hinders thrombin and other coagulation factors. During pregnancy, prothrombotic states result from deficiencies in any factor that heightens the VTE risk by about 30-fold. [72] The absolute risk for first and recurrent venous thrombus formation can be used to classify thrombophilia as high, moderate, or low. High-risk thrombophilia includes protein S, protein C, and antithrombin III deficiency. In comparison, moderate-risk thrombophilia involves factor V Leiden, factor VIII deficiency, and prothrombin gene mutations. On the other hand, low-risk thrombophilia includes hyperhomocysteinemia, factor XI, and factor IX deficiency. [73]
Moreover, factors such as the zygosity of the mutation and a previous personal or familial history have a substantial impact on the VTE risk during pregnancy. [1,69] An increased number of these criteria corresponds to a heightened risk of thrombosis. For example, in expectant women who are heterozygous for factor V Leiden and do not have a first-degree relative or a personal history of (H/O) VTE with the condition, the estimated risk of VTE is between 5 and 10/1000 deliveries. In contrast, the likelihood escalates to 10% when an individual has a personal H/O VTE, [41,74] and to 15% per 1000 deliveries when a first-degree relative is afflicted. [41,74] Lastly, the risk for homozygous expectant women is 1%-2 % if they do not have a first-degree relative or a personal H/O VTE, while those who have a history have about a 17% risk. [74]
One of the prevalent etiologies of acquired thrombophilia is antiphospholipid syndrome (APS) during pregnancy. [75] In recent times, research has yielded additional knowledge regarding APS pathogenesis. Antiphospholipid (APL) antibodies are linked to vascular thrombosis in this syndrome. In particular, the antibodies against lupus anticoagulant and anticardiolipin were frequently observed to be correlated with thromboembolic events [1,76]APS is characterized by a higher venous to arterial thrombosis (2:1) ratio and a propensity for recurrent thrombosis. Leg DVT is a prevalent clinical feature, and APS is the main initiator of its recurrence. The recurrence rate of DVT in APS was reported at 76%. [77] The increased coagulability associated with pregnancy significantly amplifies the likelihood of thrombosis in APS. Combined oral contraceptives or pregnancy are responsible for > 50% of thrombotic episodes observed in APS patients. [1,78] Even with thromboprophylaxis, numerous pregnant APS patients continue to experience thrombotic episodes, according to several studies. [1,79] The diagnosis of thrombophilia during pregnancy presents a considerable challenge because numerous laboratory assays utilized for this purpose are susceptible to change during pregnancy. [80] However, it is critical to identify women who have thrombophilia, as this information can guide prophylactic anticoagulation decisions throughout pregnancy and following delivery. Before or early in conception, a woman herself or one who has a VTE familial history, or a considerable family H/O thrombophilia, should undergo screening for inherited thrombophilia. Women who have experienced previous frequent pregnancy loss or neonatal death are also qualified for antiphospholipid syndrome screening.
Furthermore, factors such as age >35 years, multiparous (≥3), extensive varicose veins, smoking, heart failure, inflammatory bowel disease, or cancer increase DVT and VTE rates. Multiple pregnancies, preeclampsia, Caesarean section, protracted labor (>24 hours), mid-cavity or rotational surgical delivery, stillbirth, premature birth, or postpartum hemorrhage (>1 Liter or required transfusion) are obstetric risk factors. [46] Any surgery during pregnancy or the puerperium period (except for a perineal repair after delivery), post-delivery sterilization, hyperemesis gravidarum, IVF, OHSS, immobility, assisted reproductive technology, bone fracture, long-distance travel (>4 hours), and illness (e.g., pneumonia) remotely increase the VTE risk. Hence, continuous risk assessment of DVT and VTE factors during pregnancy and postpartum is crucial. VTE during pregnancy pathogenesis is summarized in Figure 1.
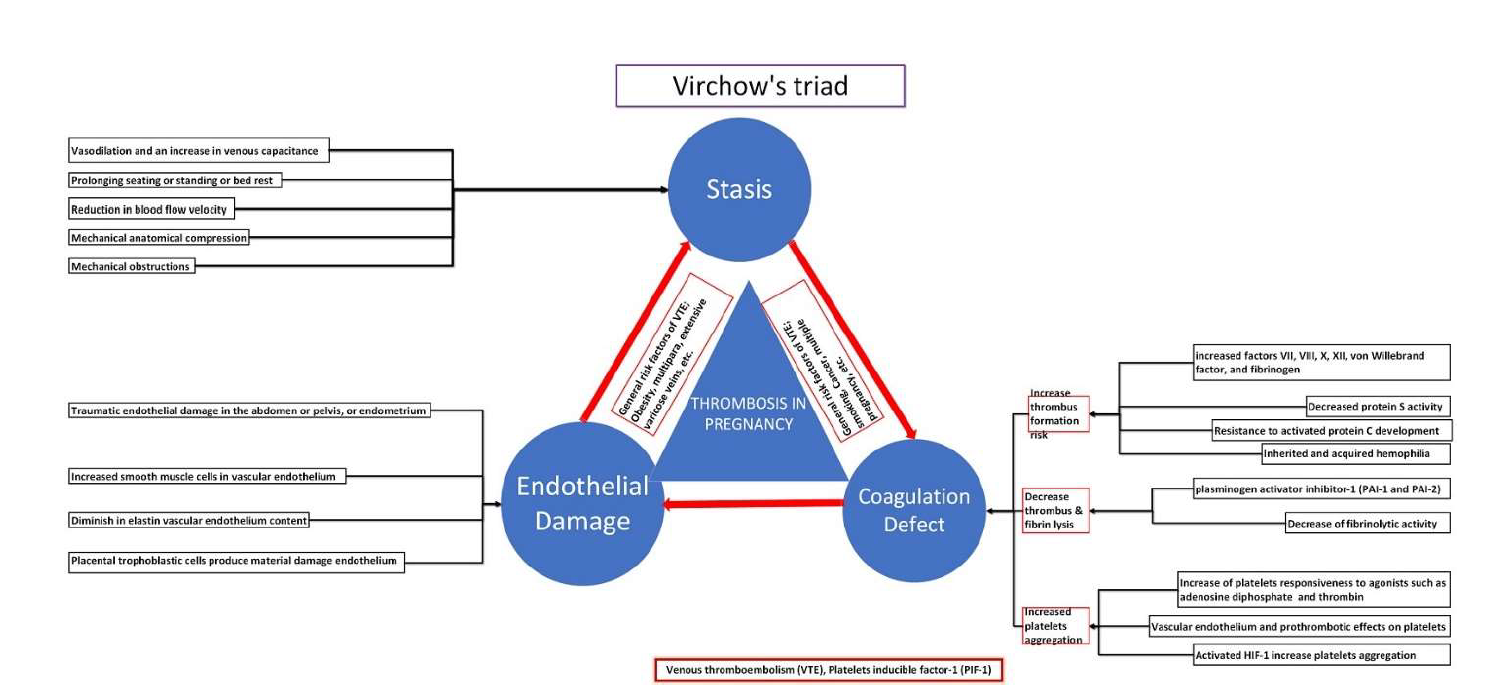
Figure 1. Pathogenesis of venous thromboembolism in pregnancy
VTE clinical awareness is essential for prompt diagnosis and therapy. DVT symptoms are nonspecific and frequently mimic symptoms of pregnancy, including lower leg swelling and discomfort. Pregnancy-associated VTE may manifest in different forms, ranging from having no symptoms to experiencing life-threatening occurrences like a major PE with unstable blood flow. Various symptoms that may indicate VTE presence in nonpregnant females, such as increased heart rate, stress, breathlessness, hemoptysis, increased respiratory rate, and swelling and discomfort in the lower extremities, are not specific and often occur during a normal, uncomplicated pregnancy. Therefore, a clinical examination is often inadequate for identifying VTE. The predominant symptoms seen in DVT during pregnancy are discomfort and edema of the lower limbs. These clinical features could or could not be associated with erythema, warmth, and soreness in the lower extremities. These features occur in around 80% of gravid women who exhibit DVT, yet the diagnosis is often overlooked. [26] During pregnancy, the occurrence of DVT is similar to that in nonpregnant women. However, there is a higher frequency of left-sided DVTs (70%-90 %), affecting iliac and proximal iliofemoral veins.
DVTs are most common in the left leg in symptomatic pregnant women. [81] Increased incidence of iliofemoral DVT in late pregnancy may be attributed to the gravid uterine compression of the left iliac vein at the anatomical location where the right iliac artery crosses. [81] In comparison, nonpregnant individuals are more susceptible to having calf distal vascular thrombi. [4] Another probable reason for this might be the increased blood pooling in the lower body venous system, especially the left iliac and femoral veins. This blood pooling is possibly because the inferior vena cava is compressed by the uterus enlargement, leading to inferior vena cava engorgement, which compresses the left iliac vein and the right iliac artery. [26,82]
The diagnostic challenge for health service providers lies in distinguishing PE presenting symptoms during pregnancy from those caused by other common benign illnesses related to pregnancy, such as gastroesophageal reflux disease, pregnancy-related physiological dyspnea, or discomfort produced because of the progressive enlargement of the uterus. PE clinical features during pregnancy characteristically include dyspnea, palpitations, and pleuritic chest pain, increased by even minimal movement. Given the acknowledgment that PE may also manifest with comparable clinical features and is a substantial contributor to maternal mortality, clinicians should have a low threshold for imaging in this group.
When VTE is clinically suspected, and delivery is not impending, low molecular weight heparin (LMWH) should be started promptly before further confirmatory investigations to confirm or deny the diagnosis. [4]. No established risk factors for pregnant women exist, unlike the Wells or Geneva guidelines for nonpregnant patients. This has made VTE diagnosis in pregnant women complex and challenging. [83] The Royal College of Obstetricians and Gynecologists (RCOG) recommends an ECG and chest X-ray for women with acute PE symptoms. [46]
Clinical guidelines suggest multiple VTE diagnostic methods for pregnant women. [84] Comprehensive leg duplex ultrasonography (DUS) is performed by applying pressure to the femoral blood vessels to visualize the iliac veins and detect DVT. [84] If the compression maneuvers of both femoral veins are positive or a thrombus in either of the iliac veins reveals DVT, treatment with LMWH should be continued, and there is no need for VQ or CTPA scan to minimize excessive radiation. Once DVT is confirmed, LMWH infusion therapy should be continued even without evidence of PE. If the entire leg DUS does not show DVT, and DVT clinic suspicion is there, a patient may be clinically monitored, and mostly LMWH infusion shall be continued.
Magnetic resonance imaging (MRI) may be used if the DUS findings are ambiguous or if high clinical suspicion persists despite the absence of iliac vein thrombi. [85] A Ventilation-perfusion (V/Q) lung scan or computed tomography pulmonary angiography (CTPA) is recommended for a pregnant woman with suspected PE and no DVT symptoms. V/Q scans or computed CTPAs depend on local guidelines, radiological availability, and physician or patient choices. If a chest X-ray is abnormal and PE is suspected, CTPA should be done instead of V/Q. [4]
In the absence of risk factors with less suspicious clinical findings, alternative diagnoses should be considered. CTPA or V/Q scan is needed to confirm PE in moderate-to-high PE suspect cases. [84] A V/Q scan with an atypical result excludes PE, but a high-likelihood result confirms a PE diagnosis. A CTPA scan is indicated when a V/Q scan is not diagnostic. It should be acknowledged that a normal CTPA scan excludes illness, and a positive result confirms PE. If the CTPA is equivocal or non-diagnostic, a V/Q scan or CTPA should be repeated. [84]
Normal D-dimer values have been thoroughly documented in non-symptomatic gravid women, and the lowest value below which PE can confidently be dismissed in symptomatic pregnant women has not been defined. RCOG recommends against routine D-dimer testing in pregnancy to investigate acute VTE because raised D-dimer levels during pregnancy are usually present. [46] Hence, the available evidence has not enabled us to determine whether average D-dimer readings and a low clinical likelihood may safely exclude PE. However, some case reports show that pregnant women who had radiologically confirmed PE but had D-dimer levels less than non-gravid cut-points. [1,86] D-dimer testing for pregnant VTE diagnosis is unclear; therefore, further multicenter projects must be planned to evaluate the significance of the D-dimer cut-point in detecting and predicting VTE in pregnancy. The diagnostic approach is summarized in Figure 2.
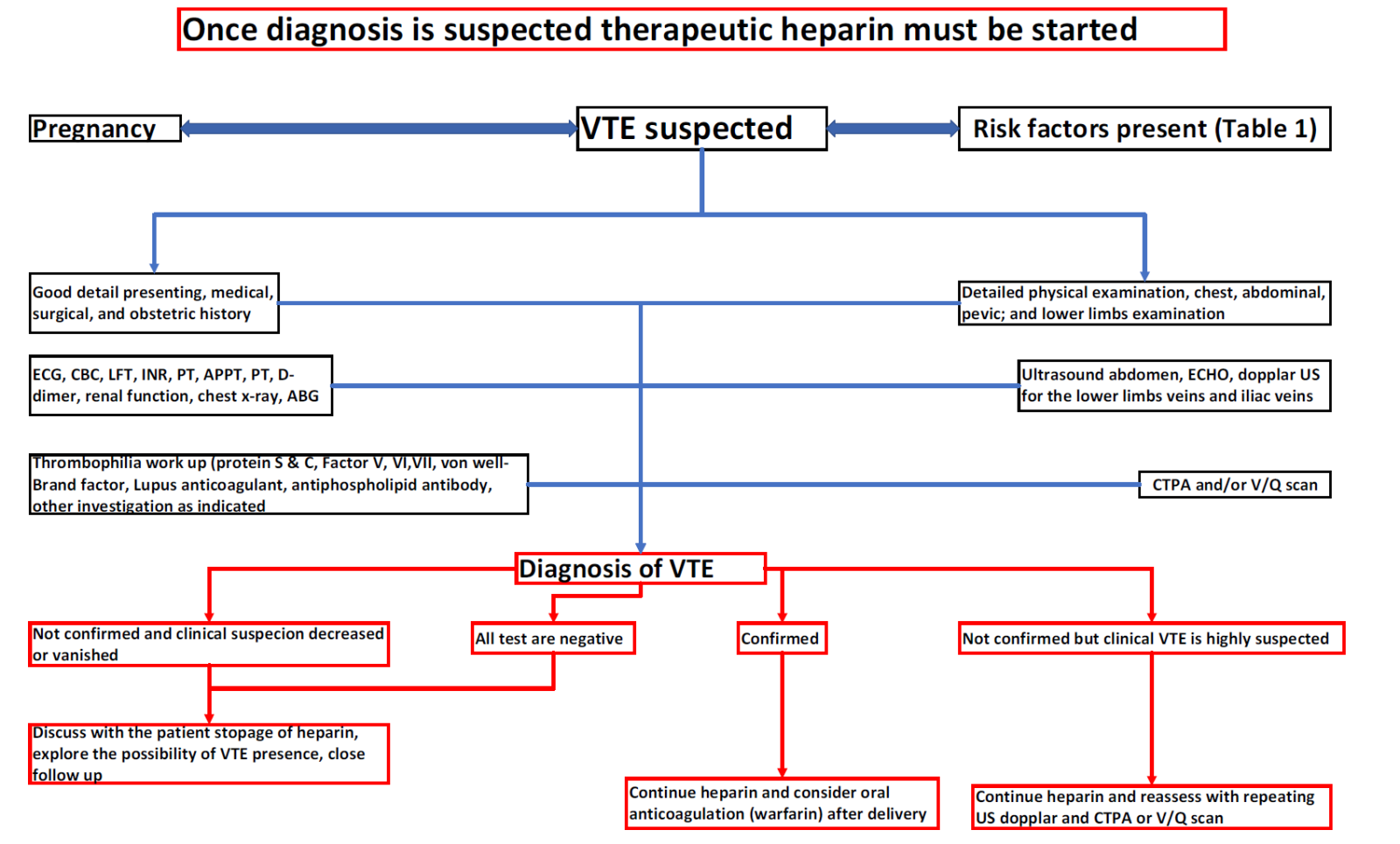
Figure 2. Scheme for venous thromboembolism diagnosis in pregnancy.
Abbreviations: Electrocardiography (ECG), echocardiography (ECHO), ultrasound (US), computed pulmonary artery angiography, ventilation perfusion (VQ) scan, prothrombin time (PT), international normalized ratio (INR), and activated partial prothrombin time (APPT).
The DVT differential diagnosis is similar in pregnant and nonpregnant women. It encompasses other disorders characterized by redness, warmth, swelling, and discomfort in the lower extremity, lower abdomen, back, or buttocks. Conditions that these presentations might include are superficial thrombophlebitis, cellulitis, Baker's cyst, lymphoedema, chronic venous insufficiency, arterial or popliteal venous aneurysm, lymph nodes enlargement compressing the veins, muscle tears, heterotopic ossification, and hemorrhage. Furthermore, DVT clinical presentation features and signs during pregnancy might resemble some typical physiological features of pregnancy, such as pain and swelling in the lower limbs. The spectrum of symptoms ranges from mild breathlessness to shock. The potential causes for symptoms like PE include heart failure, peripartum cardiomyopathy, pneumonia, pneumothorax, aortic dissection, and others. Hence, a careful history and clinical examination with critical interpretation must be thoroughly considered.
It is imperative to share the case with a senior doctor and start anticoagulant treatment for any woman who has signs or symptoms that may indicate VTE until a VTE diagnosis is ruled out. Acute VTE is preferably treated by LMWH unless there is a possibility of labor within the coming 24 hours or if the patient is very unwell. In such instances, unfractionated heparin (UFH) administration may be more suitable since protamine may readily counteract its impact.
Before initiating anticoagulation, initial blood tests, including a coagulation screen, kidney function, complete blood count, and liver function testing, are necessary. In the acute context, a thrombophilia screen is unnecessary since it will not affect the treatment plan and is often aberrant due to the thrombosis and hemostatic alterations caused by pregnancy. [87] Interpreting the data may be challenging due to pregnancy-related alterations, such as decreased protein S levels, acquired resistance to activated protein C, and elevated FVIII levels.
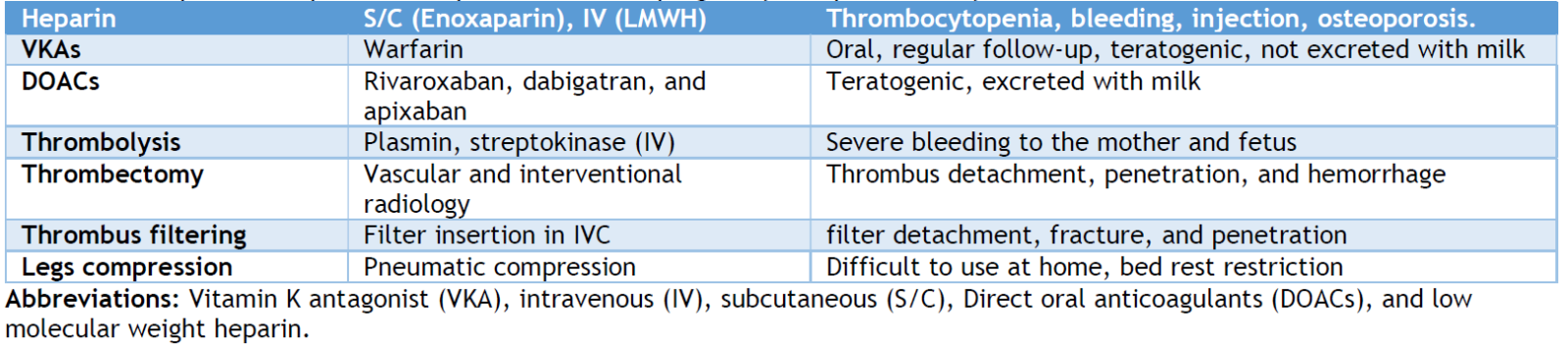
The decision to start anticoagulation therapy for pregnancy-associated VTE is difficult since anticoagulant medications may harm the fetus. [88] However, anticoagulation is the backbone of pregnant VTE treatment. The type of anticoagulant used in the treatment and prophylaxis of VTE in pregnancy depends on the thrombosis location and size, gestational age, and fetal risk. LMWHs and UFHs can be administered during pregnancy and postpartum, while warfarin is only administered postpartum as an anticoagulant. In pregnancy, LMWHs are preferable over UFH for VTE prevention and therapy. [1,89] According to randomized studies, LMWHs are as efficacious as UFH in nonpregnant women. [1] LMWHs are secreted in breast milk at low levels; therefore, nursing babies are safe. [90] Compared to UFH, LMWHs have a lower risk of bleeding, heparin-induced thrombocytopenia (HIT), osteoporosis, and allergic responses. [91] Warfarin should not be taken during pregnancy because it passes through the placenta and increases the risk of heavy bleeding, miscarriage, developmental problems, stillbirth, and neurological issues. [92] Nursing mothers may take warfarin safely because it is not excreted in breast milk. Direct oral anticoagulants (DOACs) use in pregnant and breastfeeding mothers was investigated and showed a significant risk of bleeding, which made warfarin highly recommended by guidelines. [93,94]
The use of anticoagulants during pregnancy is considered a challenge for medical practitioners. One must consider the potentially harmful effects on fetal development and the complex dosage requirements of various anticoagulant medications. Additionally, it is vital to address the challenge of managing the unexpected start of labor and the possibility of using neuraxial blockade.
Low-molecular-weight heparins (LMWH) bind to antithrombin, enhancing the antithrombin III-mediated suppression of the production and activity of coagulation factor Xa. In contrast, low molecular weight heparin (LMWH) induces reduced thrombin (factor IIa) inhibition compared to normal heparin. [95] The liver eliminates UFH, while the kidney eliminates LMWH.
Heparins usually cannot cross the placenta; hence, they will not cause bleeding to the fetus. UFHs are favored due to their user-friendly nature, low cost, consistent anticoagulation effects, and less need for monitoring. In addition, they possess a reduced susceptibility to HIT. UFH may be preferred when delivery is near due to its ability to be promptly reversed. It may be used as a substitute for LMWH in individuals with kidney impairment, since the elimination of UFH mainly occurs via the liver.
The administration of LMWH during pregnancy differs from that of nonpregnant individuals and is determined by the initial weight at presentation. International regulations might differ; thus, it is crucial to verify with local authorities. The RCOG has established body weight-based thromboprophylaxis and treatment regimens in the UK. [46] Lower dosages of LMWH (enoxaparin and dalteparin) are recommended if the creatinine clearance is below 30 ml/min or below 20 ml/min with tinzaparin. [4,46]
LMWH is subcutaneously (S/C) injected, whereas UFH is S/C or continuous intravenous (IV). The patient's weight and activated partial thromboplastin time (aPTT) help determine the first UFH dosage. LMWH is given twice a day without monitoring. [4,92]
Women with VTE who have antiphospholipid antibody syndrome, two or more congenital or acquired thrombophilia, [96] Those with any thrombophilia with recurring thromboses should receive anticoagulation for 12 months. [4,97]
Non-pharmacological therapies may treat VTE alongside anticoagulants in pregnancy. Compression stockings lower post-thrombotic syndrome, leg swelling, and discomfort. Wear them throughout the day and take them off at night. [98,99]. Early and regular ambulation and avoiding prolonged bed rest unless required are advised to reduce the VTE risk. [98] Inferior vena cava (IVC) filters may be explored in women with recurrent VTE, regardless of anticoagulant use or when anticoagulants are contraindicated. [98] However, their hazards and benefits should be carefully addressed. [100]
As a last option, thrombolytic therapy dissolves blood clots and restores blood flow using alteplase and streptokinase. Thrombolytic treatment during pregnancy might induce substantial bleeding, early labor, and placental abruption. [4,101] Few examples have been recorded of thrombolysis during pregnancy without harming the baby, and the most commonly used drug was streptokinase. [102,103] Thus, guidelines advocate thrombolytic treatment only for life-threatening conditions like PE with intractable cardiorespiratory impairment. [104]
In pregnancy, warfarin is often contraindicated. Exposure to warfarin during the first 3 months may lead to embryopathy, whereas exposure later in pregnancy can cause fetal intracranial bleeding. Warfarin may be considered for women with a metallic valve replacement who are at significant thrombosis risk during pregnancy, but only after thoroughly evaluating the potential dangers to the fetus and mother [4].
Direct oral anticoagulants (DOACs) (rivaroxaban, apixaban, and dabigatran) are not used during pregnancy due to insufficient data on their effectiveness and safety. [4] Non-heparin anticoagulants are often avoided during pregnancy, except if there is a specific reason not to use them, such as in HIT or the inability to administer injections. Danaparoid is a derivative of LMWH that does not pass through the placenta and can be used during pregnancy.
Contraindications should be considered while considering anticoagulant use. These contraindications encompass active bleeding or a significant risk of bleeding (such as placenta previa), allergy, bleeding disorder or acquired blood clotting disorder, low platelet count (platelets <75×109), HIT, recent occurrence of a stroke within the past 4 weeks, uncontrolled high blood pressure (systolic BP>200 mmHg or diastolic BP>120 mmHg), or severe liver disease (with prolonged prothrombin time). [46]
A woman with an acute large PE during pregnancy or the perinatal period may show cardiac arrest or collapse and need emergency treatment. Depending on the patient, a multidisciplinary team of skilled clinicians—obstetricians, anesthetists, and hematologists—should determine if intravenous UFH or thrombolysis is needed. It was reported that intravenous UFH is best for large PE with hemodynamic instability due to its fast onset. If thrombolysis is provided, UFH dosage may be adjusted. Thrombolysis is recommended for women with life-threatening PE and hemodynamic instability. Iv UFH should start immediately after thrombolysis. Stable UFH may be transformed into LMWH. Like nonpregnant individuals, maternal and fetal hemorrhage problems are 2–3%. [87]
In women diagnosed with antiphospholipid antibody syndrome and a documented record of three or more miscarriages of pregnancy, it is advisable to administer LMWH in combination with low-dose aspirin (75-100 mg/d) before delivery. [41,105] According to a meta-analysis, prophylactic administration of low-dose aspirin and heparin may reduce pregnancy loss in females with recurrent miscarriages and antiphospholipid antibodies by 50%. [106] Thromboprophylaxis-eligible patients with thrombophilia should commence treatment during the first trimester and maintain it for 6 weeks after delivery. [105]
In guidelines [41], postpartum prophylaxis is not recommended for emergency cesarean deliveries alone; pharmacological prophylaxis following cesarean section is recommended only in the presence of supplementary risk factors (e.g., obesity, advanced age, underlying malignancy, prolonged immobilization). [69] Patients undergoing cesarean delivery who do not have any additional risk factors for VTE are advised to consider early ambulation and/or mechanical devices (e.g., intermittent pneumatic compression).
IVF is linked with an increased VTE risk; however, the symptomatic VTE absolute incidence in unselected patients appears to be < 1% [107]. Patients who are hospitalized and have severe OHSS have been associated with increased VTE risk. [105,108] However, in cases where the patient presents with severe OHSS, prophylactic anticoagulation is not advised. [25,105]
Therapeutic LMWH can be continued throughout pregnancy without special blood tests and frequent monitoring. However, individuals with heavy weight, recurrent VTE, impaired kidney function, or antithrombin deficiency may need dose modification and anti-Xa concentration monitoring.
UFH patients must have their aPTT measured 6 hours after the loading dosage and at least once a day. The therapeutic objective for the APTT ratio is 1.5 - 2.5 times the control. Due to HIT risk, their thrombocyte count has to be checked every 3–4 days for 14 days, starting on day 4. [4]
Full anticoagulation has a substantial risk to the mother and the fetus, and a collaborative team that consists of an obstetrician, a medical team, and a hematology team follows the risky women at a consultant-led prenatal clinic.
In the general population, IVC filters are explored when anticoagulation medication is contraindicated, inefficient (recurrent VTE despite the dose of anticoagulants), or poorly tolerated due to HIT or allergy. [109] No randomized clinical studies evaluate the effectiveness or dangers of IVC filter implantation in pregnancy. Still, there is no mechanistic reason not to use, except the gravida uterus pressure effects, such as dislocation or fracture of the IVC filter. IVC filters might work during pregnancy, although insertion and removal are risky. A death rate of 0.12 - 0.3%, filter migration at >20%, and IVC perforation (5%) or filter fracture are reported complications. Thus, only females who cannot be anticoagulated or have recurring incidents despite appropriate anticoagulation should use IVC filters. [87]
VTE-affected pregnant women must be monitored to evaluate therapy response, avoid recurrence, and manage associated illnesses. Monitoring frequency depends on VTE severity, anticoagulant type, and fetal gestational age. [25] Regular coagulation and fetal monitoring are advised for pregnant women on anticoagulant medication to check fetal development and well-being. Pregnant women with VTE should also be educated about recurring VTE symptoms and advised to seek medical assistance if they develop new or deteriorating symptoms. [110] Table 2 summarizes medical and mechanical therapeutic options for venous thromboembolism in pregnancy and post-delivery.
Table 2. Therapeutic and preventive options for VTE in pregnancy and post-delivery.
INVASIVE MANAGEMENT OF VENOUS THROMBOEMBOLISM IN PREGNANCY AND POSTPARTUM UPDATE
Most pregnant VTEs resolve with anticoagulation alone. The treatment of large PE generally requires sophisticated therapy. [111] Systemic thrombolysis, catheter-directed thrombolysis, surgical procedures, and extracorporeal membrane (ECMO) are moderate and massive PE treatments. [25] Despite little evidence and opposing opinions, sub-massive PE may also be treated with modern methods. [112]
If needed, systemic thrombolysis might be performed during pregnancy. A study of systemic thrombolysis in pregnancy found 2.8% (4/141) maternal mortality and 1.4% (2/141) newborn deaths. [113] Alteplase and streptokinase transplacental transit are low and not associated with fetal coagulopathy or other abnormalities. [114] A meta-analysis of systemic thrombolysis studies in antepartum and postpartum women found a 28.4% risk for intra-abdominal bleeding or bleeding per vagina. [115] No reliable data are comparing pregnant women taking thrombolysis with women who had no thrombolysis.
IVC filters are evaluated in the general population when anticoagulation medication is contraindicated, inefficient (recurrent VTE on full-dose anticoagulation), or poorly tolerated due to HIT or allergy [109]. No randomized clinical studies evaluate the effectiveness or dangers of IVC filter implantation in pregnancy, but there is no mechanistic reason for a different method.
Other invasive pregnancy VTE treatments include surgical, percutaneous catheter, and ECMO. Among 127 peripartum women with PE, 36 had traditional thrombectomy, 7 received percutaneous venous catheter thrombectomy, and three received ECMO and anticoagulation. Surgical thrombectomy had an 86% survival rate, 20% major hemorrhage, 20% fetal mortality, and 8% preterm birth. Percutaneous thrombectomy patients survived 100%, had a 20% significant hemorrhage, and 25% fetal mortality. In 2/7 of women, this approach was inadequate and required ECMO or surgical thrombectomy. ECMO for 4–10 days in 3/127 patients was conducted, which was associated with one early birth; however, all patients lived without hemorrhage. [116] Despite a limited sample size, percutaneous and surgical thrombectomy may be better than thrombolysis, particularly during early postpartum, to prevent major postpartum bleeding. Experts should perform these surgeries in specialist locations with cardiopulmonary bypass. [117]
A multidisciplinary team approach is advised in the peripartum period, and in addition to an obstetrician with thrombosis knowledge, maternal-fetal medicine (MFM) specialists, hematologists, and physicians should be involved in the decision when and for whom anticoagulation should be started. Due to the condition's intricacy and subspecialty, a multidisciplinary team is the best plan. Huge PE during pregnancy might kill the mother and baby. Thus, life-saving interventions, including systemic thrombolysis, surgical, catheter-directed, and ECMO, should not be denied. In such circumstances, MFM, VTE specialists (vascular surgery and interventional radiology), and obstetric anesthesia must work together. Pulmonary Embolism Response Teams are becoming more common. They anticipate and coordinate treatment in severe PE situations that need expert opinions. Those teams have had significant success in severe PE in pregnancy. [118] According to Bannow et al., Postpartum patients should review their anticoagulation strategy with their primary doctor 1-2 weeks after delivery. Nurse visits and telemedicine are appropriate, but a trustworthy 24-hour contact is required when resources are limited. [119]
There is a 13% chance of recurrence for patients with pregnancy-associated VTE in subsequent pregnancies. [120] Evidence suggests that thromboprophylaxis may decrease the likelihood of VTE recurrence. Without thromboprophylaxis, the VTE recurrence rate during pregnancy was constant at 6.2% and remained constant throughout the pregnancy. According to a study composed of 159 women who had experienced at least one pregnancy following a VTE, no VTE relapses were observed in the group that received prophylaxis. [121] An additional prospective nationwide investigation examining long-term LMWH thromboprophylaxis in 326 females with VTE history discovered that the combined antepartum and postpartum subgroups had an 88% reduction in the relative risk of developing VTE. [122]
Pharmacological interventions have been shown to decrease the occurrence of VTE during pregnancy and postpartum. [41] Universal thromboprophylaxis may not be a safe course of action due to the potential for maternal bleeding, HIT, and osteoporotic fractures that may occur during heparin administration. [91] Hence, routine thromboprophylaxis is advised exclusively for women who are deemed to be at a heightened risk for VTE due to specific criteria, including a prior VTE associated with estrogen or specific inherited thrombophilia. [69] Concerning the attributes of women who are more susceptible to developing a first VTE during pregnancy or postpartum, there exists a dearth of information and disagreement, as well as inconsistency, concerning the relative impact of these risk factors on the absolute VTE risk.
A comparative analysis of LMWH and UFH in pregnant women has not yet taken place, and the data utilized for prophylaxis are derived from the nonpregnant women population. [105,123] LMWH is conventionally administered at varying doses (prophylactic, intermediate, therapeutic) during pregnancy; however, there is a lack of evidence-based consensus concerning the most effective strategy. The prophylactic 40 mg enoxaparin dose S/C is administered daily; the intermediate dose, which is 40 mg subcutaneously applied twice daily, is greater than the prophylactic dose but less than the therapeutic dose; and the therapeutic dose, which is 1mg/kg twice daily. Research examining the efficiency and safety of these strategies at various concentrations has not identified any discernible differences. [123,124] The American College of Obstetricians and Gynecologists (ACOG) and the American Society of Hematology (ASH) both endorse the utilization of prophylactic and intermediate dosing, contrary to the ASH's recommendation of prophylactic dosing. [69,105]
Generally, pharmacologic thromboprophylaxis is administered only to expectant women who are deemed to have a higher risk of developing VTE. [25,69] A H/O solitary idiopathic, estrogen-associated, or pregnancy-related VTE is linked to a > 10-fold increased risk and more than 1% absolute risk of developing VTE. [125] Females with a previous VTE in pregnancy or using oral contraceptives are more likely to encounter a recurrent VTE during pregnancy than those with a previous VTE that was unprovoked or unrelated to hormones. [126] Pregnant women who do not have at least one known risk factor for thrombophilia are not typically advised to undergo antepartum and postpartum pharmacological thromboprophylaxis [25]. While clinical risk factors elevate the likelihood of VTE during antepartum and postpartum beyond the risk observed in the general population (approximately 0.6/1000 deliveries during both periods), most individual risk factors pose a minimal absolute VTE risk of less than 1%. Prophylactic anticoagulation is advised by the ASH and ACCP guidelines for antepartum women who have unprovoked or estrogen-associated VTE, as well as for postpartum females who have had any prior VTE, regardless of the etiology. [25]
Guidelines provide differing recommendations regarding pharmacological thromboprophylaxis for thrombophilia disorders. [25,41] The specific kind of hereditary thrombophilia, VTE family history, and whether it occurs during antepartum or postpartum determine the eligibility for thromboprophylaxis in females with inherited thrombophilia.
When conducting neuraxial anesthesia with anticoagulation, vertebral canal hematoma is the most dreaded consequence. The significant and progressive vertebral canal hematoma may cause paralysis if not recognized and dealt with within 12 hours. According to the third National Audit Project, a rare one in 140,000 spinal canal hematomas causes lasting damage. [4] Non-obstetric patients had perioperative epidurals and coagulation-impairing medications in all but one instance. No obstetric instances were recorded. [127]
The indication and LMWH dosage (prophylactic or therapeutic) determine intrapartum anticoagulation treatment. A multidisciplinary approach is necessary to arrange labor and delivery for women using therapeutic or preventive anticoagulants. This plan should specify anticoagulant discontinuation. Most women should stop taking LMWH when labor begins, and medical professionals should recommend a further dose following evaluation. Delivery may be planned to maximize neuraxial block duration and reduce maternal bleeding. Despite careful preparation, some females may require emergency delivery. National and international norms govern neuraxial blockade timing and anticoagulant administration. All of them overlook pregnancy's hypercoagulability and extrapolate non-obstetric counsel to obstetric patients. In antepartum inpatients needing thromboprophylaxis, the Society for Obstetric Anesthesia and Perinatology recommends mechanical or low-dose UFH (5,000 units S/C twice daily) over LMWH or higher-dose UFH. [128] In outpatients, they recommend switching to low-dose UFH (5,000 units) S/C 12 hourly at 36 weeks or earlier from LMWH, especially in patients with obstetric morbidity or medical diseases, or women for urgent Caesarean risk or preterm delivery. [128]
The Anesthetists Association, the Obstetric Anesthetists' Association, and Regional Anesthesia UK issued a guideline that describes the timing of neuraxial blocks, their risks in pregnant females with coagulation abnormalities, and alternatives to regional anesthesia, such as general anesthesia. [129] Obstetric anesthetists must weigh the risks and benefits of withholding neuraxial block due to anticoagulation. Postponing neuraxial anesthesia by 12 or 24 hours after the previous LMWH injection is advised, depending on whether the patient receives prophylactic or therapeutic dosages. It is recommended that the administration of LMWH be delayed for 4 hours after removing the catheter and that prophylaxis be provided cautiously if the epidural catheter is used. LMWH therapy is contraindicated in patients with epidural catheters. [129] Consult the obstetric team before restarting anticoagulants after birth to account for epidural catheter removal, postpartum hemorrhage, and thrombosis.
Early identification is critical to minimize lasting harm; neurological function must be monitored, particularly after anticoagulation. Guidelines recommend monitoring obstetric neuraxial block recovery and escalation if recovery is delayed or additional symptoms occur. [130] According to these guidelines, the anesthetist should be notified if a woman cannot do a straight-leg raise during labor, and straight-leg raising must be used to screen for motor block after spinal anesthesia or epidural top-up. Each maternity unit must have a policy for care escalation according to referral pathways and local resources. [130] When the woman is not able to raise her straight leg 4 hours after the latest dose of epidural/spinal local anesthetic, the anesthetist should be informed.
VTE is a potentially avoidable cause of perinatal death. The alarming morbidity and mortality associated with VTE necessitate further research to address the knowledge and understanding deficits regarding risk factors, underlying mechanisms, and management strategies. Firstly, the current VTE prediction scores are constructed using criteria that do not encompass expectant individuals and depend on characteristics that seldom pertain to this population, such as advanced age or cancer. Hence, research endeavors focused on developing risk assessments and predictive models pertinent to expectant and postpartum women are required. Additionally, educational programs must be developed to equip medical professionals with the knowledge necessary to recognize, treat, and prevent VTE in expectant women. Second, educational sessions about DVT and PE for females during the childbearing age are essential to increase this age group's awareness and understanding of the clinical features and complications of these conditions, and further research work is required for this area. Third, current thromboprophylaxis recommendations for VTE during pregnancy and postpartum are stratified according to underlying thrombophilia or a H/O thrombotic events. Fourth, large-scale studies are required to evaluate the efficiency and safety of advanced therapeutic options for high-risk VTE; however, due to the rarity of this condition, such studies may be challenging. By addressing these voids in knowledge, clinicians will have a comprehensive understanding of VTE, which will impact the well-being of pregnant women. Furthermore, it is recommended that additional research endeavors be undertaken to validate the diagnostic algorithms for VTE in the natal and postnatal periods. These investigations should employ contemporary radiological imaging techniques and low-dose radiation.
Venous thromboembolism is a prominent cause of maternal morbidity and mortality, and it is avoidable. Clinical features may be vague. Anticoagulation using low-molecular-weight heparin is best, although the administration time and adverse effects must be considered. VTE increases fetal and maternal mortality; thus, high-risk women must be identified, diagnosed, and treated. Due to clinical and laboratory similarities between normal pregnancy and systemic thrombosis, VTE in pregnancy may be challenging to diagnose. Preventing and treating PE in obstetric patients is difficult due to the lack of evidence on anticoagulant safety and effectiveness and the possible risk to the fetus and mother. LMWH is the leading anticoagulant for at least three months during pregnancy. Under challenging circumstances like failure of various therapies, large or sub-massive PE, or acute limb-threatening DVT, thrombolysis, IVC filters, and mechanical thrombus removal should be explored despite their higher rate of complications.
AKNOWLEGMENT
We thank the Open Libyan University for the valuable support in finishing this review.
AUTHORS’ CONTRIBUTION
All authors have significantly contributed to the work, whether by conducting literature searches, drafting, revising, or critically reviewing the article. They have given their final approval of the version to be published, have agreed with the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
References
- Varrias D, Spanos M, Kokkinidis DG, Zoumpourlis P, Kalaitzopoulos DR. Venous Thromboembolism in Pregnancy: Challenges and Solutions. Vasc Health Risk Manag. 2023;19:469-84.
- Morris JM, Algert CS, Roberts CL. Incidence and risk factors for pulmonary embolism in the postpartum period. J Thromb Haemost. 2010;8(5):998–1003.
- Al-Husban N, Alnsour LN, El-Adwan Z, Saleh NA, El-Zibdeh M. Impact of Pregnancy-Related Venous Thromboembolism on Quality of Patients' Lives. Clin Appl Thromb Hemost. 2021;27:10760296211040873.
- Kearsley R, Stocks G. Venous thromboembolism in pregnancy-diagnosis, management, and treatment. BJA Educ. 2021;21(3):117-23.
- Knight M, Bunch K, Tuffnell D, Patel R, Shakespeare J, Kotnis R, Kenyon S, Kurinczuk JJ (Eds.) on behalf of MBRRACE-UK. Saving Lives, Improving Mothers' Care - Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into.
- Chang J, Elam-Evans LD, Berg CJ, Herndon J, Flowers L, Seed KA, et al. Pregnancy-related mortality surveillance--United States, 1991--1999. MMWR Surveill Summ. 2003;52(2):1-8.
- Pregnancy Mortality Surveillance System. Maternal mortality. CDC. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm. Accessed December 2024.
- Baeva S, Saxton DL, Ruggiero K, Kormondy ML, Hollier LM, et al. Identifying Maternal Deaths in Texas Using an Enhanced Method, 2012. Obstet Gynecol. 2018;131(5):762-769.
- Blondon M, Harrington LB, Righini M, Boehlen F, Bounameaux H, Smith NL. Racial and ethnic differences in the risk of postpartum venous thromboembolism: a population-based, case-control study. J Thromb Haemost. 2014;12(12):2002–2009.
- Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143:697-706.
- James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311-5.
- Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJ. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6:632-7.
- Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ. Res. 2016;118:1340-47.
- Hong J, Lee JH, Yhim HY, Choi WI, Bang SM, Lee H, et al. Incidence of venous thromboembolism in Korea from 2009 to 2013. PLoS ONE. 2018;13:e0191897.
- Danwang C, Temgoua MN, Agbor VN, Tankeu AT, Noubiap JJ. Epidemiology of venous thromboembolism in Africa: a systematic review. J. Thromb. Haemost. 2017;15:1770-81.
- Lee LH, Gallus A, Jindal R, Wang C, Wu CC. Incidence of Venous Thromboembolism in Asian Populations: A Systematic Review. Thromb Haemost. 2017;117(12):2243-60. doi: 10.1160/TH17-02-0134.
- Ceresetto JM. Venous thromboembolism in Latin America: a review and guide to diagnosis and treatment for primary care. Clinics. 2016;71:36–46.
- Vázquez FJ, Posadas-Martínez ML, Vicens J, González Bernaldo de Quirós F, Giunta DH. Incidence rate of symptomatic venous thromboembolic disease in patients from a medical care program in Buenos Aires, Argentina: a prospective cohort. Thrombosis J. 2013;11:16.
- Arab H, Abduljabbar H, Sabr Y, Bondogji N, Mosali F, Bajouh O, et al. Venous Thrombo prophylaxis in Pregnancy and Puerperium: the Saudi Algorithm. 2017; 2(3): 555590.
- Ahmed M, Akbar D, Al-Shaikh A. Deep vein thrombosis at King Abdul Aziz University Hospital. Saudi Med J. 2000;21(8):762-4.
- Sarah A Alharbi et al. Women's Awareness of Risk of DVT During Pregnancy and Puerperium: A Cross Sectional Study in Jeddah. World Family Medicine. 2020; 18(1): 187-193.
- Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG. 2001;108(1):56–60.
- McColl MD, Ramsay JE, Tait RC, Walker ID, McCall F, Conkie JA, et al. Risk factors for pregnancy-associated venous thromboembolism. Thromb Haemost. 1997;78(4):1183–1188.
- Kourlaba G, Relakis J, Kontodimas S, Holm MV, Maniadakis N. A systematic review and meta-analysis of the epidemiology and burden of venous thromboembolism among pregnant women. Int J Gynaecol Obstet. 2016;132(1):4–10.
- Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317–3359.
- James AH, Tapson VF, Goldhaber SZ. Thrombosis during pregnancy and the postpartum period. Am J Obstet Gynecol. 2005;193(1):216-19.
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-33.
- Bourjeily G, Paidas M, Khalil H, Rosene-Montella K, Rodger M. Pulmonary embolism in pregnancy. Lancet. 2010;375:500–12.
- Macklon NS, Greer IA, Bowman AW. An ultrasound study of gestational and postural changes in the deep venous system of the leg in pregnancy. Br J Obstet Gynaecol. 1997;104:191–7.
- Raia-Barjat T, Edebiri O, Chauleur C. Venous Thromboembolism Risk Score and Pregnancy. Front Cardiovasc Med. 2022;9:863612
- Rodger M, Sheppard D, Gándara E, Tinmouth A. Haematological problems in obstetrics. Best Pract Res Clin Obstet Gynaecol. 2015;29:671-84.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232(1):27-44.
- Lim A, Samarage A, Lim BH. Venous thromboembolism in pregnancy. Obstet Gynaecol Reprod Med 2016;26:133-9.
- Dado C, Levinson A, Bourjeily G. Pregnancy and Pulmonary Embolism. Clin Chest Med. 2018;39(3):525-37.
- Greer IA. Clinical Practice. Pregnancy complicated by venous thrombosis. N Engl J Med. 2015;373:540-7.
- Wik HS, Jacobsen AF, Sandvik L, Sandset PM. Prevalence and predictors for post-thrombotic syndrome 3 to 16 years after pregnancy-related venous thrombosis: a population-based, cross-sectional, case-control study. J Thromb Haemost. 2012;10(5):840-7.
- Jacobsen AF, Skjeldestad FE, Sandset PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium–a register-based case-control study. Am J Obstet Gynecol. 2008;198:233.
- Ray JG, Chan WS. Deep vein thrombosis during pregnancy and the puerperium: a meta-analysis of the period of risk and the leg of presentation. Obstet Gynecol Surv. 1999;54:265-71.
- Tepper NK, Boulet SL, Whiteman MK, Monsour M, Marchbanks PA, Hooper WC, et al. Postpartum venous thromboembolism: incidence and risk factors. Obstet Gynecol. 2014;123:987-96.
- Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MS. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370(14):1307-15
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e691S-e736S.
- Pabinger I, Grafenhofer H, Kyrle PA, Quehenberger P, Mannhalter C, Lechner K, et al. Temporary increase in the risk for recurrence during pregnancy in women with a history of venous thromboembolism. Blood. (2002) 100:1060–2.
- Iorio A, Kearon C, Filippucci E, Marcucci M, Macura A, Pengo V, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170:1710-6.
- De Stefano V, Martinelli I, Rossi E, Battaglioli T, Za T, Mannuccio Mannucci P, Leone G. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol. (2006) 135:386-91.
- Croles FN, Nasserinejad K, Duvekot JJ, Kruip MJ, Meijer K, Leebeek FW. Pregnancy, thrombophilia, and the risk of a first venous thrombosis: systematic review and bayesian meta-analysis. BMJ. 2017;359:j4452.
- Royal College of Obstetricians and Gynaecologists . vol. 37a. Green-top Guideline; 2015. (Reducing the risk of venous thromboembolism during pregnancy and the puerperium). https://www.rcog.org.uk/media/qejfhcaj/gtg-37a.pdf.
- Kevane B, Donnelly J, D'Alton M, Cooley S, Preston RJ, Ní Ainle F. Risk factors for pregnancy-associated venous thromboembolism: a review. J Perinat Med. 2014;42:417-25.
- Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost. 2008;6:905-12.
- O'Shaughnessy F, O'Reilly D, Ní Áinle F. Current opinion and emerging trends on the treatment, diagnosis, and prevention of pregnancy-associated venous thromboembolic disease: a review. Transl Res. 2020;225:20-32.
- National Guideline Alliance (UK). Risk factors for venous thromboembolism in pregnancy: Antenatal care: Evidence review N. London: National Institute for Health and Care Excellence (NICE); 2021 Aug. (NICE Guideline, No. 201.)
- Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet. 1999;353(9160):1258-65.
- Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16(2):153-68. doi: 10.1016/s1521-6926(03)00021-5.
- Dalaker K, Prydz H. The coagulation factor VII in pregnancy. Br J Haematol. 1984;56(2):233-41.
- Thornton P, Douglas J. Coagulation in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2010;24(3):339-52.
- Estellés A, Gilabert J, Andrés C, España F, Aznar J. Plasminogen activator inhibitors type 1 and type 2 and plasminogen activators in amniotic fluid during pregnancy. Thromb Haemost. 1990;64(2):281-85.
- Sheu JR, Hsiao G, Lin WY, Chen TF, Chien YY, Lin CH, et al. Mechanisms involved in agonist-induced hyperaggregability of platelets from normal pregnancy. J Biomed Sci. 2002;9(1):17-25.
- Janes SL, Goodall AH. Flow cytometric detection of circulating activated platelets and platelet hyper-responsiveness in preeclampsia and pregnancy. Clin Sci. 1994;86(6):731-39. doi: 10.1042/cs0860731.
- Aharon A, Katzenell S, Tamari T, Brenner B. Microparticles bearing tissue factor and tissue factor pathway inhibitor in gestational vascular complications. J Thromb Haemost. 2009;7(6):1047-50.
- Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2):89-94.
- Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003-8.
- Macklon NS, Greer IA, Bowman AW. An ultrasound study of gestational and postural changes in the deep venous system of the leg in pregnancy. Br J Obstet Gynaecol. 1997;104(2):191-7.
- James AH. Pregnancy-associated thrombosis. Hematology. 2009;2009(1):277-85.
- Kovacevich GJ, Gaich SA, Lavin JP, Hopkins MP, Crane SS, Stewart J, et al. The prevalence of thromboembolic events among women with extended bed rest prescribed as part of the treatment for premature labor or preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;182(5):1089-92.
- Song G, Kim J, Bazer FW, Spencer TE. Progesterone and interferon tau regulate hypoxia-inducible factors in the endometrium of the ovine uterus. Endocrinology. 2008;149(4):1926-34. doi: 10.1210/en.2007-1530.
- Abou-Ismail MY, Citla Sridhar D, Nayak L. Estrogen and thrombosis: a bench to bedside review. Thromb Res. 2020;192:40-51.
- Chen J, Khalil RA. Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog Mol Biol Transl Sci. 2017;148:87-165.
- Cerneca F, Ricci G, Simeone R, Malisano M, Alberico S, Guaschino S. Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. Eur J Obstet Gynecol Reprod Biol. 1997;73(1):31-6.
- Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114(5-6):409-14.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 197: inherited Thrombophilias in Pregnancy. Obstet Gynecol. 2018;132(1):e18–e34.
- Ridker PM. Ethnic distribution of factor V Leiden in 4047 men and women. JAMA. 1997;277(16):1305.
- Mekaj Y, Lulaj S, Daci F, Rafuna N, Miftari E, Hoxha H, et al. Prevalence and role of antithrombin III, protein C and protein S deficiencies and activated protein C resistance in Kosovo women with recurrent pregnancy loss during the first trimester of pregnancy. J Hum Reprod Sci. 2015;8(4):224-9.
- Pabinger I, Schneider B. Thrombotic risk in hereditary antithrombin III, Protein C, or Protein S deficiency. Arterioscler Thromb Vasc Biol. 1996;16(6):742-8. doi: 10.1161/01.ATV.16.6.742.
- Makris M. Thrombophilia: grading the risk. Blood. 2009;113(21):5038-9.
- Zotz RB, Gerhardt A, Scharf RE. Inherited thrombophilia and gestational venous thromboembolism. Best Pract Res Clin Haematol. 2003;16(2):243-59.
- Khare M, Nelson-Piercy C. Acquired thrombophilias and pregnancy. Best Pract Res Clin Obstet Gynaecol. 2003;17(3):491-507.
- Lechner K, Pabinger-Fasching I. Lupus anticoagulants and thrombosis. Pathophysiol Haemost Thromb. 1985;15(4):254–262.
- Khamashta MA, Cuadrado MJ, Mujic F, et al. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332(15):993-7.
- Branch DW. Antiphospholipid antibodies and pregnancy: maternal implications. Semin Perinatol. 1990;14(2):139-46.
- Ringrose DK. Anaesthesia and the antiphospholipid syndrome: a review of 20 obstetric patients. Int J Obstet Anesth. 1997;6(2):107-11.
- Bates SM. Management of pregnant women with thrombophilia or a history of venous thromboembolism. Hematology. 2007;2007(1):143-50.
- Chan WS, Spencer FA, Ginsberg JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ. 2010;182(7):657-60.
- Hull RD, Raskob GE, Carter CJ. Serial impedance plethysmography in pregnant patients with clinically suspected deep-vein thrombosis. Clinical validity of negative findings. Ann Intern Med. 1990;112(9):663–667. doi: 10.7326/0003-4819-112-9-663.
- Touhami O, Marzouk SB, Bennasr L, Touaibia M, Souli I, Felfel MA, Kehila M, et al. Are the Wells Score and the Revised Geneva Score valuable for the diagnosis of pulmonary embolism in pregnancy? Eur J Obstet Gynecol Reprod Biol. 2018;221:166-71.
- Chan WS, Rey E, Kent NE; VTE in Pregnancy Guideline Working Group, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36(6):527-53.
- Dronkers CE, Srámek A, Huisman MV, Klok FA. Accurate diagnosis of iliac vein thrombosis in pregnancy with magnetic resonance direct thrombus imaging (MRDTI). BMJ Case Rep. 2016;2016.
- To MS, Hunt BJ, Nelson-Piercy C. A negative D-dimer does not exclude venous thromboembolism (VTE) in pregnancy. J Obstet Gynaecol. 2008;28(2):222-3.
- Simcox LE, Ormesher L, Tower C, Greer IA. Pulmonary thrombo-embolism in pregnancy: diagnosis and management. Breathe (Sheff) 2015; 11(4):282-9.
- Gibson PS, Powrie R. Anticoagulants and pregnancy: when are they safe? Cleve Clin J Med. 2009;76(2):113-27.
- Krivak TC, Zorn KK. Venous thromboembolism in obstetrics and gynecology. Obstet Gynecol. 2007;109(3):761-77.
- Richter C, Sitzmann J, Lang P, Weitzel H, Huch A, Huch R. Excretion of low molecular weight heparin in human milk. Br J Clin Pharmacol. 2001;52(6):708-10.
- Greer I, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005;106(2):401-7.
- Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3):627S–644S.
- Cohen H, Arachchillage DR, Middeldorp S, Beyer-Westendorf J, Abdul-Kadir R. Management of direct oral anticoagulants in women of childbearing potential: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(8):1673-6.
- Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, et al. Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160(6):e545-e608.
- Ejaz U, Akhtar F, Xue J, Wan X, Zhang T, He S. Review: Inhibitory potential of low molecular weight Heparin in cell adhesion; emphasis on tumor metastasis. Eur J Pharmacol. 2021;892:173778.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3):401S–428S.
- Schulman S, Granqvist S, Holmström M, Carlsson A, Lindmarker P, Nicol P, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The duration of anticoagulation trial study group. N Engl J Med. 1997;336(6):393-8.
- Bagaria SJ, Bagaria VB. Strategies for diagnosis and prevention of venous thromboembolism during pregnancy. J Pregnancy. 2011;2011:206858.
- Middleton P, Shepherd E, Gomersall JC. Venous thromboembolism prophylaxis for women at risk during pregnancy and the early postnatal period. Cochrane Database Syst Rev. 2021;3(3):CD001689.
- Harris SA, Velineni R, Davies AH. Inferior vena cava filters in pregnancy: a systematic review. J Vasc Interv Radiol. 2016;27(3):354–60.e8.
- Pfeifer GW. Distribution and placental transfer of 131-I streptokinase. Australas Ann Med. 1970;19(1):17-8.
- Holden EL, Ranu H, Sheth A, Shannon MS, Madden BP. Thrombolysis for massive pulmonary embolism in pregnancy--a report of three cases and follow up over a two year period. Thromb Res. 2011;127(1):58-9.
- te Raa GD, Ribbert LSM, Snijder RJ, Biesma DH. Treatment options in massive pulmonary embolism during pregnancy; a case-report and review of literature. Thromb Res. 2009;124(1):1-5.
- Bates SM, Middeldorp S, Rodger M, James AH, Greer I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):92-128.
- Bukhari S, Fatima S, Barakat AF, Fogerty AE, Weinberg I, Elgendy IY. Venous thromboembolism during pregnancy and postpartum period. Eur J Intern Med. 2022;97:8-17.
- Empson M, Lassere M, Craig JC, Scott JR. Recurrent pregnancy loss with antiphospholipid antibody: a systematic review of therapeutic trials. Obstet Gynecol. 2002;99(1):135-44.
- Hansen AT, Kesmodel US, Juul S, Hvas AM. Increased venous thrombosis incidence in pregnancies after in vitro fertilization. Hum Reprod. 2014; 29: 611-17 29(3):611-7.
- Rova K, Passmark H, Lindqvist PG. Venous thromboembolism in relation to in vitro fertilization: an approach to determining the incidence and increase in risk in successful cycles. Fertil Steril. 2012;97(1):95-100.
- Harris SA, Velineni R, Davies AH. Inferior vena cava filters in pregnancy: a systematic review. J Vasc Interv Radiol. 2016;27(3):354-60.e8.
- Dresang LT, Fontaine P, Leeman L, King VJ. Venous thromboembolism during pregnancy. Am Fam Physician. 2008;77(12):1709–1716.
- Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011 Apr 26;123(16):1788-830.
- Kalaitzopoulos DR, Panagopoulos A, Samant S, Ghalib N, Kadillari J, Daniilidis A, et al. Management of venous thromboembolism in pregnancy. Thromb Res. 2022;211:106-113.
- Sousa Gomes M, Guimarães M, Montenegro N. Thrombolysis in pregnancy: a literature review. J Matern Fetal Neonatal Med. 2019;32(14):2418-28.
- Capstick T, Henry MT. Efficacy of thrombolytic agents in the treatment of pulmonary embolism. Eur Respir J. 2005;26(5):864-74.
- Marti C, John G, Konstantinides S, Combescure C, Sanchez O, Lankeit M, Meyer G, Perrier A. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015;36(10):605-14.
- Martillotti G, Boehlen F, Robert-Ebadi H, Jastrow N, Righini M, Blondon M. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review. J Thromb Haemost. 2017;15(10):1942-50.
- Kalaitzopoulos DR, Panagopoulos A, Samant S, Ghalib N, Kadillari J, Daniilidis A, Samartzis N, et al. Management of venous thromboembolism in pregnancy. Thromb Res. 2022;211:106-13.
- Monteleone PP, Rosenfield K, Rosovsky RP. Multidisciplinary pulmonary embolism response teams and systems. Cardiovasc Diagn Ther. 2016;6(6):662-7.
- Bannow SB, Federspiel JJ, David E. Abel, Logan Mauney L, Rachel P. Rosovsky RP, Bates SM. Multidisciplinary care of the pregnant patient with or at risk for venous thromboembolism: a recommended toolkit from the Foundation for Women and Girls with Blood Disorders Thrombosis Subcommittee. Journal of Thrombosis and Haemostasis. 2023;21(6):1432-1440.
- Brill-Edwards P, Ginsberg JS, Gent M, Hirsh J, Burrows R, Kearon C, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343(20):1439-44.
- Pabinger I, Grafenhofer H, Kyrle PA, Quehenberger P, Mannhalter C, Lechner K, et al. Temporary increase in the risk for recurrence during pregnancy in women with a history of venous thromboembolism. Blood. 2002;100(3):1060-2.
- Lindqvist PG, Bremme K, Hellgren M. Efficacy of obstetric thromboprophylaxis and long-term risk of recurrence of venous thromboembolism. Acta Obstet Gynecol Scand. 2011;90(6):648-53.
- Quinlan DJ, McQuillan A, Eikelboom JW. Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2004;140(3):175-83.
- Stephenson ML, Serra AE, Neeper JM, Caballero DC, McNulty J. A randomized controlled trial of differing doses of postcesarean enoxaparin thromboprophylaxis in obese women. J Perinatol. 2016;36(2):95-9.
- Rodger M. Pregnancy and venous thromboembolism: "TIPPS" for risk stratification. Hematology Am Soc Hematol Educ Program. 2014;2014(1):387-92.
- De Stefano V, Martinelli I, Rossi E, Battaglioli T, Za T, Mannuccio Mannucci P, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol. 2006;135(3):386-91.
- https://www.aagbi.org/sites/default/files/rapac_2013_web.pdf. Accessed March 2024.
- Leffert L, Butwick A, Carvalho B. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126:92.
- Working Party:; Association of Anaesthetists of Great Britain & Ireland; Obstetric Anaesthetists' Association; Regional Anaesthesia UK. Regional anaesthesia and patients with abnormalities of coagulation: the Association of Anaesthetists of Great Britain & Ireland The Obstetric Anaesthetists' Association Regional Anaesthesia UK. Anaesthesia. 2013 Sep;68(9):966-72.
- Yentis SM, Lucas DN, Brigante L. Safety guideline: neurological monitoring associated with obstetric neuraxial block 2020: a joint guideline by the Association of Anaesthetists and the Obstetric Anaesthetists' Association. Anaesthesia. 2020;75:913-9.
