Full HTML
The Silent Scream of Skin Cells: A Brief Review of Slow Electrical Signaling in the Epithelium
Mostafa Essam Eissa1
Author Affiliation
1 MSc (Pharmaceutical Sciences), Pharmaceutical Scientist | Certified Six Sigma Green Belt, Independent Researcher and Freelance Consultant, Former Inspector in CAPA.
Abstract
Epithelial cells, lining the skin and internal organs, play a crucial role as protective barriers and regulators of substance transport. Traditionally, these cells were not considered to employ electrical signaling for communication. However, recent investigations have unveiled that epithelial cells generate slow electrical signals, termed the "silent scream," in response to injury, thus challenging conventional views of intercellular communication. A recent experimental investigation provided compelling evidence for this phenomenon, demonstrating the ability of these cells to transmit electrical signals over considerable distances within the epithelium. The research utilized microelectrode array chips to precisely detect subtle electrical events in keratinocytes and Madin-Darby Canine Kidney (MDCK) cells, revealing spiking activity characterized by slow propagation speeds, distinct from the rapid action potentials of neurons. The mechanisms underlying this novel signaling are explored, focusing on the involvement of mechanosensitive ion channels, calcium signaling, and Adenosine triphosphate (ATP) release. Calcium ions, well-established intracellular messengers, appear to play a central role in this biological phenomenon. Integrating this newly discovered communication mode into the existing understanding of skin cell biology reveals a more intricate picture of how skin senses and responds to its environment. The implications of this finding extend to various facets of skin physiology and pathology, including wound healing, inflammation, and skin aging. In wound healing, where endogenous electric fields guide cell migration and promote repair, this unique type of electrical signaling potentially plays a crucial part. Furthermore, aberrant electrical signaling might contribute to chronic inflammatory conditions, and age-related changes in this signaling could underlie the functional decline observed in aged skin. The potential for other environmental stressors to trigger the epithelial-generated electric signals also warrants investigation. The exploration concludes by discussing potential technological applications, such as bioelectric sensors and enhanced wound healing therapies, and future research directions aimed at further elucidating the molecular mechanisms and functional roles of this non-excitable cell electrophysiology.
DOI: 10.63475/yjm.v4i1.0039
Keywords: Adenosine triphosphate (ATP), Calcium Signaling, Cell Communication, Epithelial Cells, Keratinocytes, Wound Healing.
Pages: 95-104
View: 7
Download: 17
DOI URL: https://doi.org/10.63475/yjm.v4i1.0039
Publish Date: 22-05-2025
Full Text
Intercellular communication forms the bedrock of multicellular life, enabling the fine-tuned coordination of cellular activities essential for tissue development, homeostasis, and response to environmental changes. Traditionally, this communication has been primarily understood through three main modalities: chemical signaling, involving the release and reception of molecules like hormones, neurotransmitters, and cytokines; direct cell-cell contact, facilitated by gap junctions that allow the passage of small molecules and ions between adjacent cells, and adhesion molecules that mediate physical interactions and initiate signaling cascades; and electrical signaling, predominantly associated with excitable cells such as neurons and muscle cells, characterized by rapid fluctuations in membrane potential and the flow of ions. [1-3] This established framework largely positioned electrical signaling as a specialized function, with non-excitable cells, including epithelial cells, primarily responding to chemical and mechanical cues. [1]
Epithelial cells, which constitute the linings of the skin, organs, and body cavities, serve as crucial barriers protecting the body from the external environment and regulating the transport of substances. [3] Their known repertoire of responses to external stimuli includes reacting to chemical signals like growth factors and cytokines that influence their proliferation, differentiation, and specialized functions. [4] Furthermore, epithelial cells, particularly keratinocytes in the skin, are known to be highly sensitive to mechanical stimuli, capable of sensing touch, pressure, and stretch, which can trigger intracellular signaling pathways leading to responses such as the release of adenosine triphosphate (ATP) and alterations in gene expression through mechanotransduction. [5] For instance, mechanical stress on skin cells can initiate signaling cascades involving integrins [Heterodimeric transmembrane proteins (α and β subunits)], focal adhesion kinase (FAK) [A cytoplasmic tyrosine kinase protein], extracellular signal-regulated kinase (ERK) [A member of the mitogen-activated protein kinase (MAPK) family], and phosphoinositide 3-kinase/Akt (PI3K/Akt) pathways [A complex signaling pathway involving PI3K (a lipid kinase) and Akt (a serine/threonine kinase)]. [6,7] While this mechanosensitivity and the ability of keratinocytes to release ATP upon mechanical stimulation have been recognized, the recent discovery of slow electrical signaling as a primary mode of communication in response to injury unveils a distinct and previously unappreciated aspect of their biology. [8-10] (Figure 1) depicts this complex interplay process in the simplified chart.
Recent groundbreaking research has revealed that epithelial cells possess the remarkable ability to communicate danger through slow electrical signals when injured, a phenomenon described as a "silent scream". [1] This finding challenges the traditional view of these cells as electrically passive barriers and suggests a sophisticated mechanism for coordinating cellular responses at a multicellular level within the epithelium. The analogy of a "scream" powerfully conveys the idea of a rapid, albeit temporally slower than neuronal signals, transmission of a distress signal, implying a critical role in orchestrating a collective cellular response to maintain tissue integrity upon damage. [1] This review aims to provide a comprehensive analysis of this newly discovered phenomenon, exploring the experimental evidence supporting it, the potential underlying mechanisms, its integration with existing knowledge of skin cell communication and stress responses, its implications for skin physiology and pathology, and the exciting possibilities it opens for future research and technological applications.
This article critically analyzes recent evidence revealing slow electrical signaling in epithelial cells, a departure from traditional views of intercellular communication. It synthesizes findings to characterize this novel signaling, including its kinetics, propagation, and mechanisms involving mechanosensitive ion channels, calcium signaling, and ATP release. Furthermore, the paper integrates this discovery into skin cell biology, examining its relationship with established communication pathways and implications for skin physiology and pathology, such as wound healing, inflammation, and aging. Finally, it outlines potential technological applications and future research directions to further elucidate this phenomenon.
The information presented in this brief review was gathered through a scanning search of scientific literature using relevant keywords across the internet, including several databases. The primary keywords employed included "skin cell communication," "epithelial cell signaling," "keratinocyte signaling," "skin stress response," "wound healing electrical signals," "ATP release skin," "calcium signaling skin," "mechanosensing keratinocytes," "bioelectricity skin," "epithelial action potentials," and "slow electrical signals cells." The main databases consulted were PubMed, Scopus, Web of Science, and Google Scholar, ensuring a broad search of published research, taking into consideration avoiding unnecessary scope creep.
The information presented in this brief review was gathered through a scanning search of scientific literature using relevant keywords across the internet, including several databases. The primary keywords employed included "skin cell communication," "epithelial cell signaling," "keratinocyte signaling," "skin stress response," "wound healing electrical signals," "ATP release skin," "calcium signaling skin," "mechanosensing keratinocytes," "bioelectricity skin," "epithelial action potentials," and "slow electrical signals cells." The main databases consulted were PubMed, Scopus, Web of Science, and Google Scholar, ensuring a broad search of published research, taking into consideration avoiding unnecessary scope creep. The selection of articles for this review was based on their relevance and purpose-serving the topic, with a focus on scientifically backed research papers, review manuscripts, and studies directly investigating cell communication, stress responses, and electrical signaling in skin and epithelial cells. Studies that primarily focused on neuronal or muscle cell electrical signaling without clear relevance to epithelial cells were excluded from this review. Professional scientific discussions in social media revolving around scientifically related issues and published manuscripts covering the scope of the topic were also screened and investigated. To maintain accuracy and avoid unsupported assertions, the investigation rigorously prioritized the findings of the original study by Yu and Granick (2025) as the foundation for further exploration. Information from other sources was integrated only when it corroborated or expanded upon these initial findings and was supported by evidence from reputable sources. Any discrepancies or conflicting information were carefully evaluated, and preference was given to well-established findings with strong experimental backing. All references cited within this review have been meticulously reviewed, formatted, and retrieved from their investigated websites. [1-20] A dedicated citation management software was utilized throughout the writing process to ensure the accuracy and consistency of all citations as much as possible and mitigate the risks of unintentional errors.
.png)
Figure 1. The Diagram illustrates the structure and interaction within epithelial tissue layers. It includes two subgraphs: one for the epithelial tissue with its cells connected by tight junctions and basement membrane interactions, and another for external environmental factors like mechanical stimuli and injuries that affect cellular processes.
The pivotal study that brought the "silent scream" of skin cells to light was conducted by Yu and Granick (2025) and published in the Proceedings of the National Academy of Sciences (PNAS).[1] Their research provided compelling evidence that epithelial cells, traditionally considered electrically non-excitable, exhibit traveling extracellular electric charge when injured. [21] The experimental design involved culturing human keratinocyte cells and canine Madin-Darby Canine Kidney (MDCK) cells, serving as model epithelial systems, on a custom-designed chip equipped with 60 precisely positioned microelectrodes. [1] This sophisticated setup allowed for the recording of extracellular electrical potentials with high spatial and temporal resolution. To induce localized cellular injury, the researchers employed a precise laser to "sting" individual cells within the monolayer. Subsequently, they meticulously monitored the electrical activity of the surrounding cells over time, starting approximately 10 minutes after the laser stimulus ended, and continued measurements for up to five hours. [21] Furthermore, they investigated the role of calcium ions by observing how manipulating calcium levels affected the observed electrical signals. [1]
The key findings of this groundbreaking study revealed that injured epithelial cells generated punctuated, time-dependent extracellular voltage changes, which the researchers termed "spiking activity". [21] These voltage spikes displayed depolarization, repolarization, and hyperpolarization phases, remarkably similar in shape to the action potential observed in neurons. [21] However, a significant difference was noted in the temporal scale: the duration of these epithelial spikes ranged from 1 to 2 seconds, considerably longer than the millisecond timescale characteristic of neuronal action potentials. [21] These electrical signals were also found to propagate outwards from the injury site over surprisingly long distances, reaching hundreds of micrometers and extending up to 40 times the length of a single cell. [1] The mean propagation speed of these signals was approximately 10 millimeters per second, significantly slower than the rapid transmission of signals in neuronal networks. [16] Importantly, the study demonstrated that the generation and transmission of these bioelectric signals were significantly influenced by the perturbation of mechanosensitive cationic ion channels, suggesting their crucial involvement in the phenomenon. [21] Moreover, the electrical spiking activity persisted for extended periods, with observations lasting up to five hours, indicating a sustained communication mechanism. [1] The researchers also found that calcium ion flows played a necessary role in this epithelial conversation. [1]
The discovery of this biological phenomenon was enabled by significant technological advancements, particularly the use of a high-resolution microelectrode array chip. [1] This technology allowed for the precise detection and characterization of these subtle and slow electrical events, which might have been undetectable using traditional electrophysiological techniques focused on faster signals. The researchers initially interpreted these findings as evidence for a previously unknown mode of intercellular communication in epithelial cells, triggered by injury and potentially playing a crucial role in coordinating cellular responses to maintain tissue integrity. [1] They also highlighted the potential implications of this discovery for the development of novel bioelectric medical devices and sensors for applications in wound healing and diagnostics. [1] Furthermore, the researchers suggested that this slow electrical signaling might represent a more primitive form of long-distance intercellular communication, hinting at potential evolutionary significance that warrants further investigation. [23]
The generation and propagation of electrical signals in cells are fundamentally governed by the movement of ions across the cell membrane through specialized protein channels known as ion channels. [5] These channels regulate the flow of charged ions, such as sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl-), leading to changes in the membrane potential, the electrical potential difference across the cell membrane. The aforementioned study indicated that the perturbation of mechanosensitive cationic ion channels significantly influenced the "silent scream". [17] Mechanical injury, the stimulus in their experiments, likely activates these channels, leading to an influx of positively charged ions, potentially including calcium, into the injured cell. This influx would cause a depolarization of the cell membrane, representing the initial phase of the slow electrical spike.
Calcium signaling emerges as a central mediator in this unique electrical event. [1] Calcium ions are well-established intracellular messengers involved in a vast array of cellular processes, including muscle contraction, neurotransmitter release, gene expression, and cell death. [6] In epithelial cells, calcium signaling plays crucial roles in differentiation, proliferation, and barrier maintenance. [22-24] Upon injury, intracellular calcium levels in skin cells are known to increase, contributing to membrane repair, cell migration, and inflammation. [24] The slow kinetics of the electrical spike observed in epithelial cells might be attributed to the relatively slower dynamics of calcium signaling compared to the rapid sodium and potassium fluxes that underlie neuronal action potentials. Intercellular calcium waves, which are often mediated by the release of ATP and the involvement of gap junctions, can propagate calcium signals to neighboring cells. [25-29] It is plausible that a similar mechanism contributes to the propagation of the "silent scream" across the epithelial monolayer.
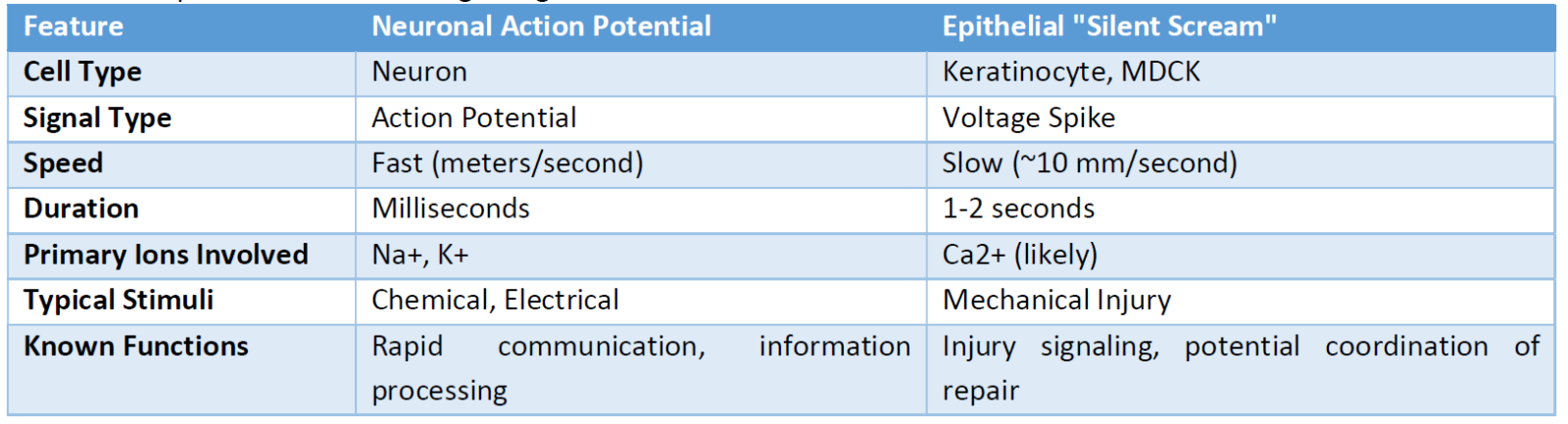
ATP, known as the energy currency of the cell, also acts as an important extracellular signaling molecule, particularly in response to stress and injury. [11] Skin cells, especially keratinocytes, release ATP in response to various stimuli, including mechanical stress. [11] Extracellular ATP can then bind to purinergic receptors [a class of cell surface receptors that are activated by purine and pyrimidine nucleotides (such as ATP) and nucleosides (such as adenosine)] on neighboring cells, triggering intracellular signaling cascades that often involve an increase in calcium influx and the release of calcium from intracellular stores. [12] It is conceivable that the mechanical injury-induced release of ATP could act as an upstream trigger for the "silent scream." The released ATP might activate purinergic receptors on adjacent cells, initiating the calcium-dependent slow electrical spiking activity. Furthermore, the sustained duration of these slow signals, lasting up to five hours, could potentially be linked to a prolonged release of ATP or a downstream signaling cascade initiated by ATP. (Table 1) shows a comparison between neuronal and epithelial signaling properties.
While the previous experiment provides significant insights, further research is needed to fully elucidate the precise molecular mechanisms underlying this newly-discovered type of signal. Identifying the specific types of mechanosensitive ion channels and calcium channels involved, as well as definitively establishing the role of ATP and other potential signaling molecules, will be crucial for a complete understanding of this phenomenon. It is also worth noting that other forms of electrical communication have been observed in non-excitable cells. For instance, GABAergic signaling has been shown to regulate electrical activity between melanoma cells and keratinocytes, indicating that electrical communication in non-neuronal cells might be more prevalent and diverse than previously appreciated. [30-38] (Table 1) summarizes the differences between the conventional neuronal and the epithelial signaling.
Table 1: Comparison of Electrical Signaling Characteristics
Keratinocytes, the predominant cell type in the epidermis, are increasingly recognized not merely as structural components forming a protective barrier but also as active participants in sensing and transmitting a diverse range of signals within the skin. [13] Their communication array is multifaceted, encompassing various mechanisms to interact with each other and with other cell types in the skin, including fibroblasts, immune cells, melanocytes, and nerve endings. These established communication methods include the release of chemical signals such as cytokines, chemokines, and growth factors that influence the behavior of neighboring cells. [7] Direct cell-cell contact is mediated by cadherins, which facilitate adhesion and initiate signaling pathways, and gap junctions formed by proteins like connexin, which allow for the direct exchange of small molecules and ions between adjacent cells. [9-40, 43] ATP signaling plays a significant role through the paracrine and autocrine activation of purinergic receptors, impacting processes like proliferation, differentiation, and inflammation. [11] Furthermore, keratinocytes communicate via the release of exosomes, small extracellular vesicles that can carry proteins, RNA, and other molecules to target cells, influencing their function. [39] Notably, keratinocytes also engage in direct communication with intraepidermal nerve fibers at the neurocutaneous unit, a process involving the release of ATP and other signaling molecules that contribute to sensory perception. [40] (Figure 2) shows chemical structures of examples of the important compounds involved in the previous processes.
The newly discovered non-excitable cell electrophysiology represents a novel and potentially complementary mode of communication within the skin epithelium. Its slow temporal scale and long-range propagation suggest that it might serve a distinct purpose compared to the faster, more localized signaling mediated by ATP or direct cell-cell contact. It is plausible that this slow electrical signaling is involved in coordinating a more sustained and widespread response to injury across the epithelial sheet, ensuring a coordinated effort in tissue repair and defense. The coordinated epithelial electrical signaling likely interacts with other established communication mechanisms in the skin. Given its calcium dependence, it could modulate or be modulated by calcium-dependent processes regulated by other signaling pathways, such as ATP-induced calcium influx and intracellular calcium release.
.png)
Figure 2: ATP chemical structure, and the protein structure of a cadherin domain and the protein structure of connexin 43 (GJA1), a key component of gap junctions in epithelial cells.
Further investigation is needed to understand how these slow electrical signals might influence the expression or activity of cytokines, adhesion molecules, or their coordination with the neuro-cutaneous unit in sensing and responding to tissue damage.
The skin, as the outermost layer of the body, serves as a critical interface with the external environment and functions as a primary stress-sensing and responding organ. [41-48] It possesses sophisticated mechanisms to detect and respond to a wide array of environmental insults, including mechanical injury, ultraviolet (UV) radiation, oxidative stress, and chemical irritants.[48] Mechanical injury, the trigger for the coordinated electrical signaling observed by Yu and Granick (2025), is a potent stressor for skin cells, initiating a cascade of stress responses. [10] These responses include the rapid release of damage-associated molecular patterns (DAMPs) like ATP, the activation of inflammatory signaling pathways, and the initiation of complex repair mechanisms.
The epithelial para-neuronal signaling, characterized by the slow electrical signals propagating through the epithelium upon injury, could function as an early warning signal, rapidly (albeit slower than neuronal signals) communicating the occurrence of mechanical damage to neighboring cells within the epithelial tissue. This early alert could trigger preemptive protective responses in the surrounding cells, such as the upregulation of stress response genes, the strengthening of intercellular junctions to maintain barrier integrity, or the release of protective molecules. Given the involvement of calcium and the potential role of ATP in the "silent scream," it is likely that this electrical signaling integrates with other established stress response pathways in skin cells. For example, it could potentially modulate the activity of transcription factors involved in the Nrf2-mediated oxidative stress response, SAPK/JNK signaling, and the unfolded protein response, thereby coordinating a comprehensive cellular defense response to injury. [48-51]
The discovery of the non-excitable cell electrophysiology in skin cells has significant implications for the understanding of various aspects of skin physiology and pathology. In the context of wound healing, where a complex sequence of cellular events is orchestrated to restore tissue integrity, the well-established role of endogenous electric fields in guiding cell migration and promoting repair suggests that the epithelial electrical activity could also play a crucial part. [52,53] The slow electrical signals might directly influence the migration of keratinocytes and other cells to the wound site, modulate the release of growth factors and cytokines involved in tissue repair, and coordinate the proliferation and differentiation of cells within the wound margin. [54-56] The sustained nature of these signals could provide a prolonged cue necessary for the completion of the multi-stage wound healing process.
Inflammation, a critical component of the skin's response to injury and infection, might also be modulated by the epithelial depolarization waves. Given the involvement of calcium and ATP, both known to participate in inflammatory processes, the electrical signals could contribute to the recruitment or activation of immune cells to the site of injury or influence the release of pro-inflammatory cytokines. [7,24] Aberrant electrical signaling in skin cells could potentially contribute to the development or maintenance of chronic inflammatory conditions.
Skin aging, characterized by a decline in various skin functions, including impaired wound healing and increased susceptibility to damage, might also be linked to alterations in the epithelial electrical activity. [50] Age-related changes in ion channel expression, calcium homeostasis, or ATP signaling could affect the efficiency or characteristics of this electrical communication, contributing to the observed functional decline in aged skin. Furthermore, the potential for other environmental stressors, such as UV radiation, chemical irritants, or pathogens, to trigger the epithelial intercellular electrical communication warrants investigation. [44, 51, 57-61] Different types of stress might elicit distinct electrical signaling patterns, suggesting a nuanced communication system within the epithelium that allows for specific and tailored cellular responses to various challenges.
The discovery of the epithelial electrical activity opens up promising possibilities for the development of novel technological applications. The unique characteristics of these slow electrical signals, such as their specific voltage pattern and relatively slow kinetics, could be exploited to develop advanced wearable or implantable bioelectric sensors. [1] Such sensors could enable the early detection of tissue damage or injury, real-time monitoring of wound healing progress, and potentially even the diagnosis of skin conditions based on altered electrical signaling patterns. The slow kinetics of the signal might necessitate the development of specialized sensor technologies optimized for this specific temporal scale.
Furthermore, understanding the parameters of the natural epithelial depolarization waves could pave the way for the development of enhanced wound healing therapies. Targeted electrical stimulation therapies that mimic or modulate these endogenous signals might accelerate and improve wound healing outcomes. [1] Optimizing stimulation parameters such as voltage, frequency (if any), and duration based on the characteristics of the natural signal could lead to more effective interventions. The development of bioelectronic bandages capable of delivering controlled electrical signals to wound sites could also revolutionize wound care. [22] A deeper understanding of the precise role of the epithelial intercellular electrical communication in different phases of wound healing could allow for targeted electrical interventions at specific time points to maximize therapeutic efficacy. Additionally, the potential for using electrical signals to enhance the transdermal delivery of therapeutic agents for treating skin conditions and the utilization of this knowledge to create more physiologically relevant in vitro skin models for drug testing and research represent other promising avenues for technological advancement. [6,55]
The discovery of the non-neuronal signaling marks an exciting new chapter in the exploration of skin biology and cellular communication, and it also raises numerous intriguing questions that warrant further investigation. A primary focus of future research should be to definitively identify the complete molecular machinery involved in this biological process. This includes pinpointing the specific types of mechanosensitive ion channels, calcium channels, and other ion channels responsible for the generation and propagation of the slow electrical signals. [17] Furthermore, the potential involvement of other signaling molecules, such as ATP, neurotransmitters, neuropeptides, and other signaling factors known to be present in the skin, needs to be thoroughly explored. [11]
Another crucial area of research will be to investigate the intracellular signaling cascades that are activated in neighboring cells upon receiving the effector response. Understanding the downstream cellular responses triggered by this electrical signal will provide insights into its functional significance. Exploring whether similar slow electrical signaling occurs in other types of epithelial tissues beyond the skin is also an important direction for future studies. While the initial findings in the recent experiment were primarily based on in vitro models, conducting in vivo studies in living organisms is essential to confirm the existence and functional relevance of this biological phenomenon in a more complex physiological environment. Comparative studies across different species could also provide valuable insights into the evolutionary origins and conservation of this signaling mechanism. [2] Given that electrical communication appears to be an ancient mechanism present in early metazoans, understanding its conservation and adaptation in different organisms could reveal fundamental principles of tissue physiology. [63,64] Finally, investigating how this biological process is altered in various skin diseases and conditions could uncover its potential as a diagnostic biomarker or a therapeutic target.
The discovery of slow electrical signaling, or the "silent scream," in skin epithelial cells represents a significant paradigm shift in our understanding of intercellular communication in non-excitable tissues. This phenomenon, characterized by neuron-like voltage spikes with considerably slower kinetics and propagation speeds, suggests a novel mechanism by which epithelial cells can rapidly communicate injury over relatively long distances. The involvement of mechanosensitive ion channels and calcium signaling appears central to this process, with potential contributions from ATP release and purinergic signaling. Integrating this new mode of communication into the existing framework of skin cell biology reveals a more complex and dynamic picture of how the skin senses and responds to its environment. The potential implications of this discovery are vast, ranging from the development of innovative bioelectric sensors and enhanced wound healing therapies to a deeper understanding of the pathophysiology of skin aging and inflammatory conditions. Future research aimed at elucidating the precise molecular mechanisms, functional roles in various physiological contexts, and evolutionary conservation of the unique electrical signaling promises to further expand our knowledge and potentially lead to transformative applications in medicine and bioengineering.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
References
- SciTechDaily. Scientists Detect Silent “Scream” of Skin Cells for the First Time [Internet]. Monrovia (CA): SciTechDaily; 2025 [cited 2025 Apr 3]. Available from: https://scitechdaily.com/scientists-detect-silent-scream-of-skin-cells-for-the-first-time/
- ScienceDaily. Slow, silent 'scream' of epithelial cells detected for first time [Internet]. Rockville (MD): ScienceDaily; 2025 Mar 17 [cited 2025 Mar 21]. Available from: https://www.sciencedaily.com/releases/2025/03/250317160337.htm
- The Scientist. Epithelial Cell Signaling Helps Maintain Tissue Integrity [Internet]. Midland (ON): The Scientist Magazine®; [date unknown] [cited 2025 Mar 21]. Available from: https://www.the-scientist.com/epithelial-cell-signaling-helps-maintain-tissue-integrity-69253
- Patterson AM, Watson AJ. Deciphering the complex signaling systems that regulate intestinal epithelial cell death processes and shedding. Frontiers in immunology. 2017 Jul 18;8:841. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2017.00841/full
- Laly AC, Sliogeryte K, Pundel OJ, Ross R, Keeling MC, Avisetti D, et al. The keratin network of intermediate filaments regulates keratinocyte rigidity sensing and nuclear mechanotransduction. Science Advances. 2021 Jan 27;7(5):eabd6187. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC7840118/
- Hunter-Featherstone E, Young N, Chamberlain K, Cubillas P, Hulette B, Wei X, Tiesman JP, Bascom CC, Benham AM, Goldberg MW, Saretzki G. Culturing keratinocytes on biomimetic substrates facilitates improved epidermal assembly in vitro. Cells. 2021 May 12;10(5):1177. Available from: https://www.mdpi.com/2073-4409/10/5/1177
- Chung H, Oh S, Shin HW, Lee Y, Lee H, Seok SH. Matrix stiffening enhances DNCB-induced IL-6 secretion in keratinocytes through activation of ERK and PI3K/Akt pathway. Frontiers in immunology. 2021 Nov 11;12:759992. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2021.759992/full
- Shutova MS, Boehncke WH. Mechanotransduction in skin inflammation. Cells. 2022 Jun 25;11(13):2026. Available from: https://www.mdpi.com/2073-4409/11/13/2026
- Martino F, Perestrelo AR, Vinarský V, Pagliari S, Forte G. Cellular Mechanotransduction: From Tension to Function. Front Physiol [Internet]. 2018 Jun 27 [cited 2025 Mar 21];9:824. Available from: https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2018.00824/full
- Chien WC, Tsai TF. The Pressurized Skin: A Review on the Pathological Effect of Mechanical Pressure on the Skin from the Cellular Perspective. Int J Mol Sci [Internet]. 2023 Oct 13 [cited 2025 Mar 21];24(20):15207. Available from: https://www.mdpi.com/1422-0067/24/20/15207
- Barr TP, Albrecht PJ, Hou Q, Mongin AA, Strichartz GR, Rice FL. Air-Stimulated ATP Release from Keratinocytes Occurs through Connexin Hemichannels. PLoS One [Internet]. 2013 Feb 21 [cited 2025 Mar 21];8(2):e56744. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0056744
- Azorin N, Raoux M, Rodat‐Despoix L, Merrot T, Delmas P, Crest M. ATP signalling is crucial for the response of human keratinocytes to mechanical stimulation by hypo-osmotic shock. Cell Calcium [Internet]. 2011 May [cited 2025 Mar 21]; 20(5):401-7.
- Riding A, Pullar CE. ATP Release and P2Y receptor signaling are essential for keratinocyte galvanotaxis. Journal of cellular physiology. 2016 Jan;231(1):181-91.
- Burnstock G, Knight GE. Cell culture: complications due to mechanical release of ATP and activation of purinoceptors. Cell and tissue research. 2017 Oct;370:1-1. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5610203/
- Ryu Y, Wague A, Liu X, Feeley BT, Ferguson AR, Morioka K. Cellular signaling pathways in the nervous system activated by various mechanical and electromagnetic stimuli. Frontiers in Molecular Neuroscience. 2024 Oct 4;17:1427070. Available from: https://www.frontiersin.org/journals/molecular-neuroscience/articles/10.3389/fnmol.2024.1427070/full
- Colombia One. Scientists Find The Silent 'Scream' of Human Skin For The First Time [Internet]. Colombia One; 2025 Mar 18 [cited 2025 Mar 21]. Available from: https://colombiaone.com/2025/03/18/silent-scream-human-skin/
- Starr M. ScienceAlert. Scientists Found The Silent 'Scream' of Human Skin For The First Time [Internet]. ScienceAlert; 2025 Mar 18 [cited 2025 Mar 21]. Available from: https://www.sciencealert.com/scientists-found-the-silent-scream-of-human-skin-for-the-first-time
- Rhode Island PBS. Cells Lining Organs Can Generate Electricity When Injured, Offering New Wound Treatment Possibilities [Internet]. Providence (RI): Rhode Island PBS Foundation; [date unknown, possibly 2025 Mar] [cited 2025 Mar 21]. Available from: https://www.ripbs.org/news-culture/health/cells-lining-organs-can-generate-electricity-when-injured-offering-new-wound-treatment-possibilities
- AZoLifeSciences. Calcium-Mediated Electrical Communication in Epithelial Cells [Internet]. Manchester (UK): AZoNetwork; 2025 Mar 18 [cited 2025 Mar 21]. Available from: https://www.azolifesciences.com/news/20250318/Calcium-Mediated-Electrical-Communication-in-Epithelial-Cells.aspx
- Yu SM, Granick S. Electric spiking activity in epithelial cells. Proceedings of the National Academy of Sciences. 2025 Mar 25;122(12):e2427123122
- Li JY, Ren KK, Zhang WJ, Xiao L, Wu HY, Liu QY, et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem cell research & therapy. 2019 Dec;10:1-7
- Sitaraman S. The Scientist. Skin Cells Create Neuron-Like Electric Signals [Internet]. Midland (ON): The Scientist Magazine®; [date unknown, likely 2025 Mar] [cited 2025 Mar 21]. Available from: https://www.the-scientist.com/skin-cells-create-neuron-like-electric-signals-72815
- Chung F. Cells lining your skin and organs can generate electricity when injured − potentially opening new doors to treating wounds: « We found that wounded epithelial cells can propagate electrical signals across dozens of cells that persist for several hours. [Internet]. Reddit; 2024 Jun 27 [cited 2025 Mar 21]. Available from: https://www.reddit.com/r/EverythingScience/comments/1jf8m56/cells_lining_your_skin_and_organs_can_generate/
- Leon Guerrero PA, Rasmussen JP, Peterman E. Calcium dynamics of skin-resident macrophages during homeostasis and tissue injury. Molecular Biology of the Cell. 2024 Dec 1;35(12):br26
- Bonsignore G, Martinotti S, Ranzato E. Wound Repair and Ca2+ Signalling Interplay: The Role of Ca2+ Channels in Skin. Cells. 2024 Mar 11;13(6):491
- Bikle DD. Role of vitamin D and calcium signaling in epidermal wound healing. Journal of Endocrinological Investigation. 2023 Feb;46(2):205-12
- Sipka T, Peroceschi R, Hassan-Abdi R, Groß M, Ellett F, Begon-Pescia C, et al. Damage-Induced Calcium Signaling and Reactive Oxygen Species Mediate Macrophage Activation in Zebrafish. Front Immunol [Internet]. 2021 Feb 23 [cited 2025 Mar 21];12:636585. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2021.636585/full
- Kallergi E, Nikoletopoulou V. Coping with the calcium overload caused by cell injury: ER to the rescue. Cell Stress [Internet]. 2021 Oct 2 [cited 2025 Mar 21];5(10):108-110. Available from: http://www.cell-stress.com/researcharticles/2021a-chandra-cell-stress/
- Handly LN, Wollman R. Wound-induced Ca2+ wave propagates through a simple release and diffusion mechanism. Molecular biology of the cell. 2017 Jun 1;28(11):1457-66
- Makenzy M. Mechanisms of Altered Calcium Signaling via ATP-activated P2Y2 Receptor in Breast Cancer Cells [Dissertation on the Internet]. Baltimore (MD): University of Maryland, Baltimore; 2024 [cited 2025 Mar 21]. Available from: https://archive.hshsl.umaryland.edu/handle/10713/22624
- Jiang LH, Mousawi F, Yang X, Roger S. ATP-induced Ca2+-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cellular and Molecular Life Sciences. 2017 Oct;74:3697-710
- Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, et al. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A [Internet]. 2016 May 17 [cited 2025 Mar 21]; 99(15):9840-5.
- Deng K, Luo R, Chen Y, Liu X, Xi Y, Usman M, et al. Electrical Stimulation Therapy–Dedicated to the Perfect Plastic Repair. Advanced Science.:2409884.
- Ceriani F, Pozzan T, Mammano F. Critical role of ATP-induced ATP release for Ca2+ signaling in nonsensory cell networks of the developing cochlea. Proceedings of the National Academy of Sciences. 2016 Nov 15;113(46):E7194-201
- Yamamura K, Ohno F, Yotsumoto S, Sato Y, Kimura N, Nishio K, et al. Extracellular ATP Contributes to Barrier Function and Inflammation in Atopic Dermatitis: Potential for Topical Treatment of Atopic Dermatitis by Targeting Extracellular ATP. International journal of molecular sciences. 2024 Nov 15;25(22):12294
- Silva-Vilches C, Ring S, Mahnke K. ATP and Its Metabolite Adenosine as Regulators of Dendritic Cell Activity. Front Immunol [Internet]. 2018 Nov 14 [cited 2025 Mar 21];9:2581. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2018.02581/full
- Inoue K, Denda M, Tozaki H, Fujishita K, Koizumi S, Inoue K. Characterization of multiple P2X receptors in cultured normal human epidermal keratinocytes. Journal of investigative dermatology. 2005 Apr 1;124(4):756-63.
- Tagore M, Hergenreder E, Perlee SC, Cruz NM, Menocal L, Suresh S, et al. GABA Regulates Electrical Activity and Tumor Initiation in Melanoma. Cancer Discov [Internet]. 2023 Oct 4 [cited 2025 Mar 21];13(10):2270-2291. Available from: https://aacrjournals.org/cancerdiscovery/article/13/10/2270/729368/GABA-Regulates-Electrical-Activity-and-Tumor
- Coy-Dibley J, Jayaraj ND, Ren D, Pacifico P, Belmadani A, Wang YZ, et al. Keratinocyte-Derived Exosomes in Painful Diabetic Neuropathy. bioRxiv [Preprint on the Internet]. 2024 Aug 22 [posted]; [cited 2025 Mar 21]. Available from: https://www.biorxiv.org/content/10.1101/2024.08.21.608803v1
- Christoph E, Britz S, Philine D, Klein T, Sauer M, Christian S, et al. Interaction of human keratinocytes and nerve fiber terminals at the neuro-cutaneous unit. eLife [Internet]. 2023 Mar 3 [cited 2025 Mar 21];12:e77761. Available from: https://elifesciences.org/articles/77761
- Rosenbach T, Czarnetzki BM. Signal transduction pathways in keratinocytes. Experimental Dermatology. 1992 Apr;1(2):59-66
- Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. Journal of Investigative Dermatology. 2001 May 1;116(5):633-40.
- Isaac C, Paggiaro AO, Aldunate JL, Herson MR, Altran SC, Mônica Beatriz M, et al. Role of keratinocytes in wound contraction: an impact assessment using a model of collagen matrix populated with fibroblasts. Revista Brasileira de Cirurgia Plástica. 2011;26:402-6
- Xu X, Yu C, Xu L, Xu J. Emerging roles of keratinocytes in nociceptive transduction and regulation. Front Mol Neurosci [Internet]. 2022 Sep 8 [cited 2025 Mar 21];15:982202. Available from: https://www.frontiersin.org/journals/molecular-neuroscience/articles/10.3389/fnmol.2022.982202/full
- Baumbauer KM, DeBerry JJ, Adelman PC, Miller RH, Hachisuka J, Lee KH, et al. Keratinocytes can modulate and directly initiate nociceptive responses. eLife [Internet]. 2015 Sep 18 [cited 2025 Mar 21];4:e09674. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4576133/
- Francie M, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Thiago A, et al. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. eLife [Internet]. 2018 Mar 15 [cited 2025 Mar 21];7:e31684. Available from: https://elifesciences.org/articles/31684
- Lumpkin EA, Marshall KL, Nelson AM. The cell biology of touch. J Cell Biol [Internet]. 2010 Oct 18 [cited 2025 Mar 21];191(2):237-48. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2958477/
- Liu HM, Cheng MY, Xun MH, Zhao ZW, Zhang Y, Tang W, et al. Possible mechanisms of oxidative stress-induced skin cellular senescence, inflammation, and cancer and the therapeutic potential of plant polyphenols. International Journal of Molecular Sciences. 2023 Feb 13;24(4):3755.
- Chen Y, Lyga J. Brain-Skin Connection: Stress, Inflammation and Skin Aging. Inflamm Allergy Drug Targets [Internet]. 2014 Jun [cited 2025 Mar 21];13(3):177-190. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4082169/
- Papaccio F, Caputo S, Bellei B. Focus on the contribution of oxidative stress in skin aging. Antioxidants. 2022 Jun;11(6):1121.
- QIAGEN. Toxicology, Cellular Stress & Stress Response [Internet]. Hilden (Germany): QIAGEN; [Date unknown] [cited 2025 Mar 21]. Available from: https://geneglobe.qiagen.com/us/knowledge/pathways/cellular-activity-metabolism-and-homeostasis-pathways/toxicology-cellular-stress-stress-response
- Qian H, Shan Y, Gong R, Lin D, Zhang M, Wang C, et al. Mechanism of action and therapeutic effects of oxidative stress and stem cell-based materials in skin aging: Current evidence and future perspectives. Frontiers in Bioengineering and Biotechnology. 2023 Jan 9;10:1082403.
- Preetam S, Ghosh A, Mishra R, Pandey A, Roy DS, Rustagi S, et al. Electrical stimulation: a novel therapeutic strategy to heal biological wounds. RSC advances. 2024;14(44):32142-73.
- Reid B, Zhao M. The electrical response to injury: molecular mechanisms and wound healing. Advances in wound care. 2014 Feb 1;3(2):184-201.
- Hussain MA. The importance of advanced modalities in wound healing: a focus on the effects of electrical stimulation. Wound Pract Res [Internet]. 2020 Dec [cited 2025 Mar 21];28(4):168-172. Available from: https://journals.cambridgemedia.com.au/wpr/volume-28-number-4/importance-advanced-modalities-wound-healing-focus-effects-electrical-stimulation
- Messerli MA, Graham DM. Extracellular Electrical Fields Direct Wound Healing and Regeneration. Biol Bull [Internet]. 2011 Aug [cited 2025 Mar 21];221(1):79-92.
- Cheah YJ, Buyong MR, Mohd Yunus MH. Wound Healing with Electrical Stimulation Technologies: A Review. Polymers (Basel) [Internet]. 2021 Oct 31 [cited 2025 Mar 21];13(21):3790. Available from: https://www.mdpi.com/2073-4360/13/21/3790
- Tai G, Tai M, Zhao M. Electrically stimulated cell migration and its contribution to wound healing. Burns Trauma [Internet]. 2018 Jul 3 [cited 2025 Mar 21];6:22.
- Lee SE, Lee SH. Epidermal keratinocyte polarity and motility require Ca2+ influx through TRPV1. J Cell Sci [Internet]. 2013 Dec 1 [cited 2025 Mar 21];126(Pt 23):5526-37. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3817792/
- Byun KA, Kim HM, Oh S, Son KH, Byun K. Radiofrequency Irradiation Attenuated UVB-Induced Skin Pigmentation by Modulating ATP Release and CD39 Expression. International Journal of Molecular Sciences. 2023 Mar 14;24(6):5506.
- Sumners DP, Green DA, Mileva KN, Bowtell JL. Increases in inspiratory neural drive in response to rapid oscillating airflow braking forces (vibration). Respiratory physiology & neurobiology. 2008 Feb 29;160(3):350-2.
- Ho CL, Yang CY, Lin WJ, Lin CH. Ecto-nucleoside triphosphate diphosphohydrolase 2 modulates local ATP-induced calcium signaling in human HaCaT keratinocytes. PloS one. 2013 Mar 11;8(3):e57666.
- Burgstahler R, Koegel H, Rucker F, Tracey D, Grafe P, Alzheimer C. Confocal ratiometric voltage imaging of cultured human keratinocytes reveals layer-specific responses to ATP. American Journal of Physiology-Cell Physiology. 2003 Apr 1;284(4):C944-52.
- Moroz LL, Romanova DY. Selective Advantages of Synapses in Evolution. Front Cell Dev Biol. 2021 Aug 20;9:726563. PMID: 34490275; PMCID: PMC8417881.
