Full HTML
Cerebrospinal Fluid Leakage Post-Lumbar Puncture: A Narrative Review
Elmukhtar Habas1, Khaled Alarbi2, Amnna Rayani3, Ala Habas4, Eshrak Habas4, Kalifa Farfar5, Aml Habas6, Almehdi Errayes7, Khawla Alzaitoni8, Gamal Alfitori7
Author Affiliation
1 Professor/Senior Consultant, Department of Medicine, Hamad General Hospital, Prof of Internal Medicine, Qatar University, Doha-Qatar, Open Libyan University, Libya
2 Consultant, Department of Medicine, Hamad General Hospital, Doha-Qatar
3 Professor/Senior Consultant, Tripoli Children Hospital, Open Libyan University, Tripoli-Libya,
4 Resident, Department of Medicine, Tripoli Central Hospital, University of Tripoli, Tripoli-Libya
5 Consultant, Department of Medicine, Alwakra General Hospital, Alwakra-Qatar
6 Specialist, Tripoli Children Hospital, Open Libyan University, Tripoli-Libya
7 Senior Consultant, Department of Medicine, Hamad General Hospital, Doha-Qatar
8 Specialist, Pediatric and Child Healthcare Department, Rayhan Medical Complex, Doha-Qatar.
Abstract
Cerebrospinal fluid (CSF) is critical in maintaining brain interstitial fluid balance and providing hydromechanical protection. Lumbar puncture (LP) is a common invasive procedure for obtaining CSF samples to evaluate central nervous system infections and cancers and measure intracranial pressure. While LP is generally considered safe, it is associated with both minor and major complications. Post-LP meningitis occurs in approximately 50% of spinal anesthesia cases and 9% of diagnostic LPs. Additionally, over 70% of diagnostic LPs result in minor bleeding, which can lead to serious outcomes such as spinal epidural hematoma, nerve damage, or paralysis. Significant consequences of LP include headaches and hearing loss; however, other rare complications, such as cerebral herniation and CSF leak syndrome, must be considered carefully. This review synthesizes findings from multiple studies published in PubMed, Google Scholar, and Scopus, highlighting the need for further research on the complications and interventions related to this commonly performed procedure.
DOI: 10.63475/yjm.v4i1.0074
Keywords: Cerebrospinal Fluid (CSF), Lumbar Puncture (LP), Complication of LP, Headache, CSF Leak.
Pages: 105-116
View: 18
Download: 22
DOI URL: https://doi.org/10.63475/yjm.v4i1.0074
Publish Date: 22-05-2025
Full Text
Lumbar puncture (LP), popularly known as a "spinal tap," is a frequently conducted procedure that involves the extraction of cerebrospinal fluid (CSF) for analysis. Heinrich Quincke created it in the late 19th century. [1] LP is the definitive diagnostic standard procedure for meningitis, subarachnoid bleeding, and other neurological diseases. Intracranial pressure can be measured via LP, and drugs or diagnostic substances can be administered. [2, 3] LP helps to diagnose infectious (encephalitis, meningitis), inflammatory (MS, Guillain-Barré syndrome), oncologic, and metabolic diseases. Furthermore, LP may be required to inject chemotherapy agents and antibiotics intrathecally. [2] The main complications of LP are back pain, headache, nausea, and vomiting. Brain herniation, infection, hematomas, and death occur, but they are rare. [4-6] CSF persistent leakage is a rare complication; however, it must be suspected when the patient develops persistent headaches and back pain. [7, 8] CSF leakage can be prevented by excluding increased intracranial pressure, avoiding multiple LP trials and excessive sampling, using a small LP needle pore, and the proper patient positioning during and after LP. [9] This review will review and update indications, contraindications, and complications, including the post-LP leak syndrome. Updated PubMed, Scopus, Google, and Google Scholar articles published between January 2018 and December 2024 were studied to achieve this aim.
CSF is a plasma ultrafiltrate found in the brain's ventricles and cranial and spinal subarachnoid regions. [10] A 150-ml adult CSF volume is distributed as 125 ml in the subarachnoid areas and 25 ml in the ventricles. [10] The choroid plexus secretes most CSF, with other sources having a less clear role. Its daily secretion in adults ranges from 400 to 600 ml, according to the method utilized to measure the volume and the person's age, sex, plus other factors. [10] Approximately 60%-75% of the formed CSF is synthesized into the 3rd and 4th ventricles by telachoroidea and lateral ventricle choroidal plexus cells (CP). [10]
The CPs are simple, highly specialized cuboidal epithelial cells embedded between the brain ventricle and ependymal cells. Plasma is filtered by cuboidal epithelium around the fenestrated capillaries. [11] CP cells feature abundant apical microvilli. They form a blood-CSF barrier via tight junctions to regulate CSF composition. [10] Due to continual secretion, a typical young adult renews CSF four to five times daily. A decreased CSF turnover may cause metabolite build-up in aging and neurological disorders. CSF composition is closely monitored, and any deviation may aid diagnosis. [10]
The blood-CSF barrier is a semipermeable membrane that regulates the brain environment. Ions and tiny molecules, such as vitamins and minerals, can enter the CSF, but cells and proteins cannot. The Osmotic water channel-1 channels allow water to move through the CP epithelium. CP epithelial cells may produce or transport brain-necessary substances into the CSF, which may not cross the blood-CSF barrier. A 5-mV lumen-positive voltage potential exists across CP epithelial cell membranes. The electrical potential difference pushes sodium, bicarbonate ions, and chloride from plasma to the CSF, generating an osmotic gradient that draws water in. [11]
CSF contains more sodium, magnesium, and chloride than plasma but less calcium and potassium. CSF contains fewer cells, immunoglobulins, and other proteins than plasma. The blood-CSF barrier prevents cells from crossing; however, modest quantities of leucocytes may be present. CSF cell count is usually below 5 cells/ml. [10] CSF composition remains consistent despite blood structure and flow variations, creating a steady intraventricular milieu necessary for neuronal function. [11]
CSF secretion by the choroidal cells occurs in two stages. The first phase involves plasma passive filtration from the choroidal capillaries into the choroidal interstitial compartment, driven by a pressure gradient. The second stage involves active transfer from the interstitium milieu to the ventricle's lumens via the choroidal epithelium, using carbonic anhydrase and membrane ion transport proteins.
Cytoplasmic carbonic anhydrase facilitates the synthesis of hydrogen and bicarbonate ions from water and carbon dioxide. The carrier proteins in the basolateral membranes of choroidal cells facilitate the exchange of hydrogen and bicarbonate ions for sodium and chloride ions. ATP-dependent ion pumps in the apical membrane transport chloride, bicarbonate, sodium, and potassium ions to the ventricular lumen. Water transport, mediated by aquaporin-1 in the apical membrane, adheres to the osmotic gradient difference created by these pumps, which creates a difference in osmolarity. [12] The sodium-potassium-2-cholride cotransporter located in the apical membrane facilitates bidirectional ion transport and controls the composition and secretion of CSF.
Interestingly, the CPs release growth factors that likely influence the subventricular zone, perhaps facilitating the healing of tissue alterations associated with hydrocephalus. They produce vitamins B12, B1, and C, folate, nitric oxide, arginine vasopressin, and β2-microglobulin. Approximately 20% of the peptides in CSF originate from the brain, and the concentration of these peptides diminishes during the CSF transitions from the ventricles to the subarachnoid regions. [13] Furthermore, extrachoroidal secretion of CSF originates from cerebral capillaries and extracellular fluid across the blood-brain barrier. This route appears to have negligible function under physiological conditions. CSF may also arise from the ependymal epithelium, which is controlled by growth factors and neuropeptides and may be modified by ependymal alterations, particularly those generated by ventricular dilation.
To ensure brain stability, CSF is released continually with an unchanged composition. The repetitive systolic pulse wave in the choroidal arteries propels the CSF down the neuroaxis from secretion to absorption. Breathing frequency, posture, jugular vein venous pressure, physical exertion, and time of day are less important CSF flow factors. [14]
CSF moves from the rostral to the caudal direction within the ventricular system. Starting in the lateral ventricles, CSF passes through the interventricular foramina to reach the third ventricle, travels via the cerebral aqueduct to the fourth ventricle, and finally enters the subarachnoid space at the base of the brain through the median aperture (foramen of Magendie). Once in the subarachnoid area, CSF circulates gently in various directions, maintaining its composition. It then flows around the brain and spinal cord within this space. CSF ultimately exits the subarachnoid area through arachnoid villi along the superior sagittal venous sinus, intracranial sinuses, and spinal nerve roots.
The arachnoid villi protrude through the dura mater into venous sinus lumens. The arachnoid villi and a 3–5 mmHg pressure gradient difference between the venous sinus and subarachnoid space draw CSF into the venous outflow system. CSF may enter the lymphatic system through the nasal cribriform plate or spinal nerve roots. CSF clearance depends on posture and pressure differentials. [10, 14]
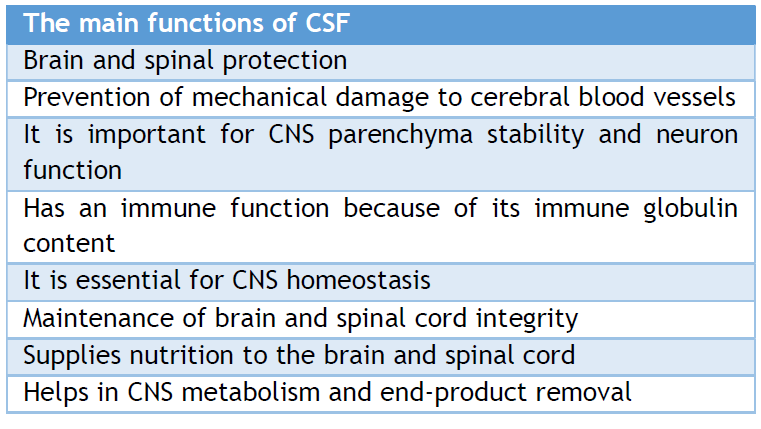
Beyond its protective role, CSF is crucial in the spinal cord and brain tissues, and it aids in removing waste products from the central nervous system. CSF provides hydromechanical protection to the neuroaxis in two ways: it acts as a cushion for the brain against the skull and shock absorber, and it creates buoyancy for the spinal cord and brain, reducing their effective weight from 1,500 grams to 50 grams. This significant weight reduction minimizes mechanical brain tissue and cerebral artery damage. Moreover, the CSF is essential for maintaining the balance of brain interstitial fluid and ensuring the stability of brain parenchyma, which is necessary for proper neuronal function. Furthermore, CSF has immune-protective and homeostasis functions.
The CP-CSF- extracellular space barrier ( ECSB) nexus supplies most of the nutrients to the brain. Brain-needed substances travel from the blood via the CP, CSF, and ECSB to their brain locations. CSF helps remove brain metabolic waste such as glycosylated proteins, peroxidation products, excess neurotransmitters, ventricular debris, germs, viruses, and unneeded chemicals. Aging and several neurodegenerative illnesses cause the accumulation of superfluous molecules, impairing brain function. The disturbance of brain physiology caused by CSF hydrodynamics or composition shows the relevance of CSF functioning. [10, 11, 14] Table 1, summarizes the main functions of CSF.
Table 1. the main functions of CSF
LP is frequently used to diagnose severe headaches and disorders of the central nervous system (CNS). During LP, the patient is positioned in either a lateral recumbent position with flexed knees, chest, and head or a sitting position with forward flexion to maximize the intervertebral space. A sterile spinal needle is carefully inserted between the vertebrae. Typically, the needle is introduced between the L3/4 or L4/5 level and passes swiftly into the subarachnoid area. To enhance success rates and mitigate the risk of complications, imaging techniques, such as fluoroscopy or ultrasound, guide needle placement and entrance direction. Upon the flow of cerebrospinal fluid through the needle, the CSF is collected in four sterile containers. [15] The collected CSF can subsequently be analyzed for abnormal or elevated components to facilitate diagnosis. (Figure 1) shows the position and typical site for LP.
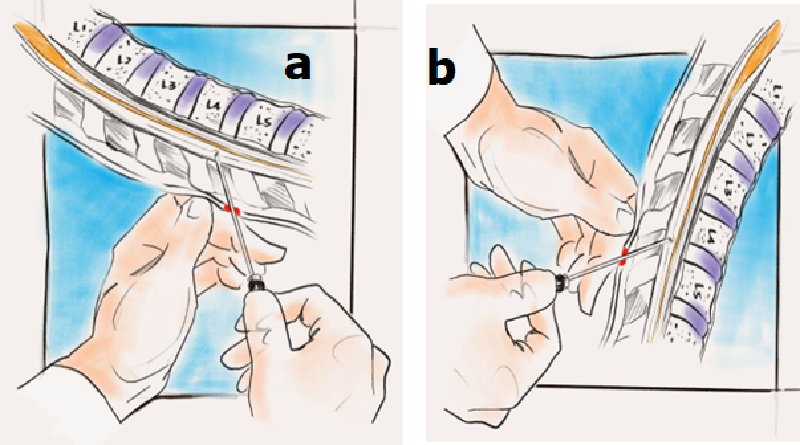
Figure 1: Site of the patient who underwent LP. a. Lateral side position. b. Sitting position
CSF is typically a transparent, colorless liquid. A yellow-orange hue in the CSF, known as xanthochromia, results from the breakdown of erythrocytes and may indicate a subarachnoid hemorrhage (SAH). Elevated levels of immunoglobulins could suggest either an autoimmune disease or systemic infection. Examination of the CSF has a high sensitivity and specificity for determining the presence of bacterial, viral, and fungal meningitis. The presence of blood indicates hemorrhagic CSF, which increases the possibility of SAH or trauma. In traumatic LP, CSF fluid is usually reddish in the first bottle; then, the color becomes less in the 2nd bottle. In the 3rd and 4th bottles, the CSF becomes clear and transparent. Meanwhile, in SAH, the color does not change. Furthermore, other findings, such as abnormal cells and proteins, may indicate malignancy or infections.
The chemical composition of the CSF primarily includes proteins and glucose. The usual range for CSF protein in adults is between 23 to 38 mg/dL. [16] The broadest reported range of normal CSF protein concentrations is 9 to 93 mg/dL. [17] Elevated CSF protein levels have been associated with advanced age, male sex, spinal stenosis, diabetes mellitus, and hypertension. [17] In adults, the standard CSF glucose concentration is reported to be 2.5 to 4.4 mmol/L. [18]
CSF glucose levels fluctuate with serum glucose levels and exhibit significant hourly variations. [19] The normal CSFglucose to serum glucose ratio level falls between 0.5 and 0.8. [20, 21] The normal CSF contains no cells. Nevertheless, it is considered within normal limits in adults to have up to five leukocytes and five erythrocytes when CSF is obtained without trauma to blood vessels during LP introduction. [22] A count exceeding three polymorphonuclear leukocytes/ microliter is abnormal for adult patients. The CSF becomes cloudy when it contains 200 leukocytes or 400 erythrocytes/µL. A CSF sample might appear bloody if it has ≥ 6000 erythrocytes/microliter. [16]
More than 70% of diagnostic LPs result in minor bleeding due to trauma from the LP needle, thereby contaminating the CSF sample. [23] This is especially common with multiple needle insertions, difficult anatomy, or bleeding disorders. However, an intra-axial bleeding source may also be responsible for this finding. If blood is not clear in the 3rd or 4th tube, intra-axial bleeding rather than traumatic LP is the most likely cause. Other indicators of intra-axial bleeding include a higher ratio of leukocytes to erythrocytes in the CSF than in the peripheral blood. Furthermore, xanthochromia usually indicates that bleeding started > 2 hours before the LP. Bleeding from a traumatic tap is usually small and may be treated conservatively; however, persistent bleeding can result in spinal epidural hematoma, nerve damage, or paralysis. Xanthochromia (yellow or pink CSF supernatant after centrifugation) is due to hemoglobin breakdown into oxyhemoglobin (pink) and then into bilirubin, causing this type of CSF discoloration. Visual identification of xanthochromia is possible [24], but laboratory spectrophotometry investigations may be more sensitive to hemoglobin after erythrocyte breakdown from oxyhemoglobin to methemoglobin to bilirubin. [25]
LP is necessary and very helpful for diagnosing CNS infections. It can also help diagnose SAH, demyelinating diseases, Guillain-Barré syndrome, other inflammatory disorders, and CNS cancers. However, with the widespread availability of neuroimaging techniques such as CT and MRI, there are limited indications for urgent diagnostic LP.
Table 2: Indications of lumbar puncture.
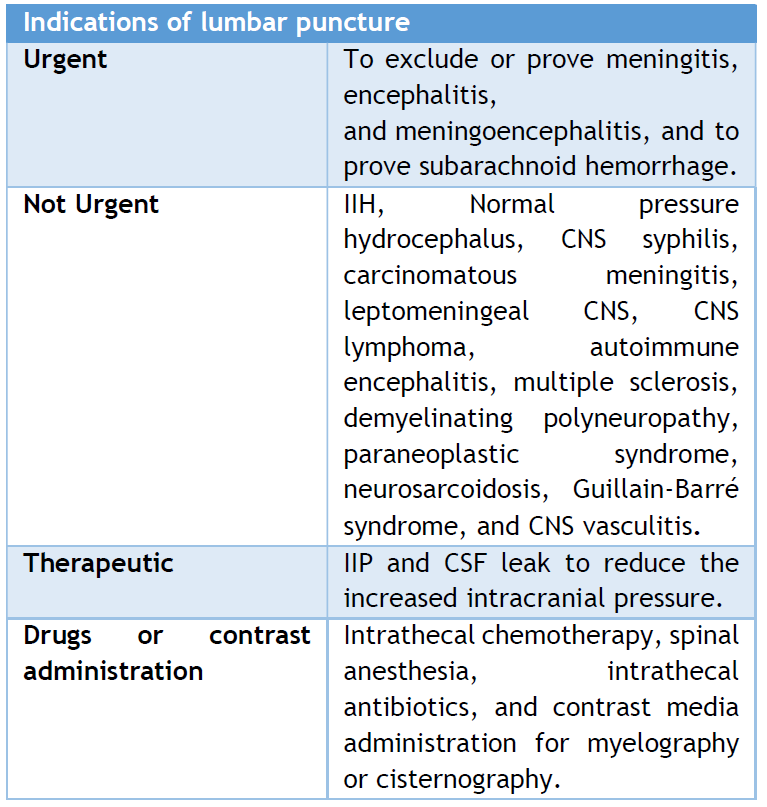
Nevertheless, urgent LP remains indicated for diagnosing suspected infectious meningitis, encephalitis, and SAH in patients with negative CT scan results. [15, 26]
The primary indication of LP is in diagnosing or excluding meningitis in patients presenting with a combination of symptoms, including fever, altered mental status, headache, or meningism. (Table 2) presents the common indications for LP.
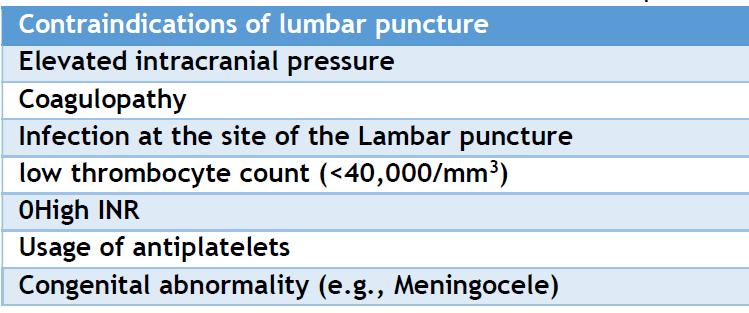
LP is contraindicated in instances of significantly elevated intracranial pressure, coagulopathies, and dermal infections at the site of the LP. In clinical practice, practitioners often request a brain CT or MRI before a lumbar puncture to rule out elevated intracranial pressure. [27] Neuroimaging is strongly advised when a physician identifies symptoms or indicators of elevated intracranial pressure, including altered consciousness, focal neurological deficits, immunocompromised status, new-onset seizures, and malignancy. [28, 29] Clinicians' vigilance is therefore essential.
Recent research indicated that platelet counts below 40,000/mm3 and international normalized ratio (INR) beyond 1.5 are contraindications. [3] The platelet count must exceed 50,000/mm3, and the INR should be below 1.5. [29] Clinicians should exercise caution with anticoagulants and antiplatelet agents because they represent relative contraindications, albeit with little supporting evidence. [30] Soft tissue infection at the lumbar puncture site is a contraindication. [3] Performing the procedure in the presence of infection at or near the puncture site may lead to a new CNS infection, compromising the reliability of CSF investigatory results. Myelomeningocele, a developmental defect, is also a contraindication. [27] (Table 3) represents the contraindication of lumbar puncture.
Table 3: Summarizes the contraindications of lumbar puncture
Although LP is generally considered a safe procedure, it can lead to rare but serious complications. These include infection, nerve root pain, bleeding, cerebral herniation, or intermittent or continuous CSF leak. Headache is a commonly reported complication following the LP, which typically begins during the first day post-LP and usually resolves by the tenth day. [2] LP complications might occur even when standard precautions are applied.
Infection
When the standard septic procedures are used, infection following LP is rare. Although rare, meningitis can occur as a complication of LP. An analysis of 179 post-LP meningitis cases documented in literature from 1952 to 2005 revealed that 50% of the incidents were associated with spinal anesthesia, while only 9% resulted from diagnostic LP. The primary causative organisms identified were Streptococcus spp.. Salivarius (30%), alpha-hemolytic Strept. (11%), Staph. aureus (9%), Pseudomonas aeruginosa (8%), and Streptococcus spp..viridans (2%).(31) Post-LP meningitis caused by staphylococci, Pseudomonas, and other gram-negative bacilli has been linked to contaminated equipment, solutions, or suboptimal techniques. [32] Other studies suggest that post-LP meningitis may stem from aerosolized oropharyngeal fluids of individuals attending or performing the procedure, particularly given that many causative organisms are found in the oral cavity and upper respiratory tract. [31, 33, 34] Few cases of discitis or vertebral osteomyelitis following LP have been reported. Most of these cases are attributed to normal skin flora, such as Cutibacterium species and coagulase-negative staphylococci. [35-37] These complications are believed to result from direct bacterial inoculation of the vertebrae.
Bleeding
More than 70% of diagnostic LPs result in minor bleeding due to needle trauma. [23] This is especially common with multiple needle insertions, difficult anatomy, or bleeding disorders. However, an intra-axial bleeding source may also be responsible for this finding. Persistent bleeding can result in spinal epidural hematoma, nerve damage, or paralysis.
In the absence of bleeding danger, spinal epidural hematoma seldom compromises the spinal cord or cauda equina. [38] A 2016 literature analysis found 35 incidences of spinal epidural hematoma following LP. [39] Patients with thrombocytopenia or other bleeding problems or who took anticoagulant medication before or after LP are at risk of bleeding. Seven of 342 patients (2%) who received anticoagulant medication after LP had spinal hematoma, five of whom developed paraparesis. [40] A previous study found that 47% of 21 LP-related spinal hematoma cases had coagulopathy. [41] Thus, all LP patients with neurologic symptoms, even those without coagulopathy, should be suspected of spinal hematoma. Other bleeding risk factors, including violent or repetitive LP trials, may enhance the likelihood of spinal epidural hematoma following LP.
Hidden bleeding makes spinal hematoma diagnosis difficult; Thus, a strong index of suspicion is required. After LP, patients with chronic back pain or neurologic symptoms (e.g., paralysis, impaired sensation, or incontinence) need immediate spinal MRI for spinal hematoma exclusion. [42] Patients with severe or worsening neurologic impairments need immediate laminectomy and blood removal. Hematoma decompression must be done quickly to prevent neurologic damage. [43, 44] Patients with mild symptoms can be managed conservatively with careful monitoring and dexamethasone administration to reduce the risk of damage. [45]
Headache
One of the most prevalent consequences of LP is headache, which affects 10–70% of patients. CSF leaking from the dura and tension on pain-sensitive tissues create post-LP headaches. Patients usually have frontal or occipital headaches within 24 to 48 hours of the surgery, which worsen in upright posture and improve in the supine position. Nausea, vomiting, dizziness, tinnitus, and vision abnormalities may occur.
In addition to post-LP headaches, other intracranial hypotension syndromes may occasionally develop. Rare post-LP sequelae include cranial nerve dysfunction, subdural hematoma, and cerebral venous thrombosis due to CSF leakage-induced intracranial hypotension. Intracranial hypotension compresses the cranial nerves, dural bridging veins, and venous sinuses.
Cranial nerve dysfunction
Horizontal diplopia may have resulted from paralysis of cranial nerve VI (abducens nerve), causing ocular misalignment. LP may cause unilateral or bilateral abducens palsy. [46, 47] Abducens nerve palsies after LP are usually accompanied by post-LP headaches. Most individuals recover within days or weeks. Oculomotor (cranial nerve III) and combined abducens and trochlear nerve (cranial nerve IV) palsy may cause diplopia in rare circumstances. [48, 49]
LP may cause hearing loss in 10-50% of individuals during spinal anesthesia, with fewer than 25% aware of the condition. [50, 51] It might be unilateral or bilateral and occur without headaches. Hearing loss is normally temporary, although spinal anesthesia, inadvertent dural puncture, and diagnostic LP may cause a years-long CSF loss. [9] Although unconfirmed, cerebral hypotension may cause traction of the vestibulocochlear nerve (cranial nerve VIII), causing hearing loss. Previous studies have linked larger needle sizes [52] and cutting needles to hearing loss. [53] The hearing loss is usually corrected using an epidural blood patch. [54]
Subdural hematoma
The most probable mechanism is due to the sudden reduction of intracranial pressure, rupturing dural bridging veins, causing subdural hemorrhage after LP. LP was linked to subdural hematoma in large retrospective cohort studies. [55-57] Subdural hematoma may cause headaches. Positional post-LP headaches develop while sitting or standing and improve or resolve with recumbency. Subdural hematoma may induce headaches unrelated to position, nausea, vomiting, neck discomfort, and focal neurologic symptoms such as hemiparesis. In almost 22 million birthing patients who underwent neuraxial anesthesia, the incidence of subdural hematoma was 1.5/100,000, compared to 309/100,000 for post-LP headache. [56] Furthermore, LP bleeding may cause a spinal subdural hematoma. [58] Due to the danger of irreversible neurologic impairment from cauda equina syndrome, patients with spinal subdural hematoma may require neurosurgical intervention. [59]
Cerebral vein thrombosis
After LP, intracranial hypotension and brain CSF pressure drop may lead to cerebral venous thrombosis, usually in the context of post-LP headache and subdural hematoma. [60] In retrospective cohort studies, LP independently predicts cerebral venous thrombosis. [57, 61] In a retrospective cohort study of over 1 million patients who received neuraxial anesthesia for childbirth, 4800 developed post-dural puncture headache (PDPH). PDPH patients had a significantly higher incidence of subdural hematoma and cerebral venous thrombosis (0.3% versus 0.02%). [57]
Cerebrospinal-venous fistula
The CSF-Venous fistula directly links the draining paraspinal vein and spinal subarachnoid space, facilitating the CSF's fast passage into the venous system. CSF is often reabsorbed at the spinal nerve roots by arachnoid villi, which are controlled by vacuoles. CSF leakage due to cerebrospinal fluid fistulas (CVFs) is uncontrolled, reducing CSF volume and lowering CSF tension. Although the thoracic spine spaces are the predominant site of CVFs, other sites, such as the lumbar region, may occur. It is more common in obese females with increased intracranial pressure. [62, 63] MRI-myelography is better than CT-myelography for diagnosing CSF-venous fistula. [64] Epidural blood patch, injection of fibrin glue, and fistula surgical ligation are the recommended therapies for severe cases. [65]
Cerebral herniation
The most significant consequence of LP is cerebral herniation. Cardiorespiratory collapse, unconsciousness, and death may occur. An intracranial mass lesion, cerebral edema, or obstructive hydrocephalus may increase ICP, contraindicating an LP and requiring separate testing and treatment. In 1959, 15 (12%) of 129 patients with elevated ICP who underwent LP had an unsatisfactory result after 48 hours. [66] Seven (13%) of 55 SAH patients developed neurologic worsening during or shortly after an LP, six of whom had cerebral herniation. [67] Another 1533 individuals with acute bacterial meningitis experienced clinical worsening of 47 (3%) following LP. [68] LP's influence on these outcomes might be difficult to determine in individuals at risk of herniation due to SAH or other brain disorders that raise ICP.
Rare complications
Other rare cerebrovascular syndromes that are associated with dural puncture include RCVS (Reversible cerebral vasoconstriction syndrome) [69], PRES (Posterior reversible encephalopathy syndrome) (70, 71), Subarachnoid and intraventricular bleeding. [72] The RCVS and PRES occurrences are associated with preeclampsia or eclampsia. Radicular symptoms and low back discomfort occur in 13% of patients with transitory electrical-type pain in one leg during the treatment. [73] Sustained radicular symptoms or damage are unusual. [74]
Post-LP, one-third of patients have localized back discomfort that may last several days but rarely persists for more than a week. [73] EBP usage does not seem to reduce persistent back pain in patients with post-LP headaches. [75, 76] Epidermoid spinal cord tumor is an uncommon LP complication that may appear years after the treatment. Most occurrences include children aged 5 -12 who had an LP in infancy; however, it has also been documented in adults. [76, 77] Epidermoid tissue injected into the spinal canal during LP, without or with a poorly fitted style, may induce this. This LP consequence may be prevented by using spinal needles with tight-fitting styles. [78] CSF leakage is another rare complication of LP. Complications are summarized in (Table 4).
Table 4: Summarizes the complications associated with LP.
.png)
Dura mater holes or tears can lead to CSF leaks. The outermost layer of the meninges that protects the central nervous system connects the epidural and subarachnoid space and, sometimes, to the skin, allowing CSF to leak. [7] CSF leaks may produce low CSF pressure, causing neck pain, headaches, ear ringing, and loss of smell and taste in some cases. A severe or continuous leak may reduce cerebral blood flow and increase the risk of direct brain parenchyma injuries due to the fluid cushion function. Open subarachnoid space communication with CSF leak may cause life-threatening CNS infections such as meningitis. Thus, CSF leak symptoms require additional assessment and care.
Structures compromised by craniofacial trauma account for 80% of CSF leaks. The remaining 4% of CSF leaks result from various causes, including iatrogenic lesions, 16%. CSF leaks can occur post-LP (especially after multiple attempts), epidural anesthesia, lumbar-abdominal shunt placement, and spinal surgery. [79] Types of CSF leaks include traumatic, iatrogenic, and spontaneous. [80, 81] The most common symptoms associated with Low CSF pressure (orthostatic) include headaches (92%), nausea (54%), and neck discomfort (43%). [82]
The most common invasive procedure performed by physicians and neurologists is LP, which may cause post-lumbar puncture syndrome, leading to headaches. Posture-dependent headache is the main symptom of this syndrome due to prolonged spontaneous CSF leakage. The headache frequency ranges from 1% to 30%, which depends on the LP approach. A 22-gauge, Whitacre atraumatic or Sprotte needle reduces the post-LP syndrome/headache risk. [83] Most patients with spontaneous low CSF pressure syndrome are identified weeks to months later. This condition should be considered in all patients with persistent headaches.
Spinal CSF leaks are now acknowledged to arise from three principal mechanisms: meningeal diverticula, CSF-venous fistulas, and ventral dural hole. [84] Meningeal diverticula are regions of dural dehiscence that allow the leptomeninges to protrude through the dural defect, resulting in a fragile, rupture-prone outpouching. Certain diverticula include extensive meningeal lacerations that facilitate quick CSF egress, whereas others result in gradual CSF seepage during the Valsalva maneuver. Ventral dural rips are mostly induced by calcified disc protrusions or pointed endplate osteophytes that sever the dura, resulting in a longitudinal rupture. Ventral tear leaks often occur rapidly, leading to substantial epidural CSF accumulations.
Beta-2 transferrin has been demonstrated as a valuable test for diagnosing CSF leaks and even for resolution. [85, 86]
Beta-trace protein, synthesized in the leptomeninges and secreted in CSF, can facilitate the diagnosis of a CSF leak. The assay is cost-effective and demonstrates specificity and sensitivity comparable to beta-2 transferrin assays, rendering it a viable diagnostic option. [87, 88] However, its availability is limited in most regions of the world. Furthermore, the utility of the test may be constrained in cases of meningitis, as low concentrations of beta-trace protein can be detected in blood, and meningitis may result in decreased beta-trace protein levels in CSF. [88] Another possible test is a glucose test, as CSF has about the same amount of glucose as blood.
The most likely imaging and diagnostic tests include computerized tomography (CT) scans, magnetic resonance imaging (MRI) scans, digital subtraction angiography, myelography, and cisternography.
Managing a CSF leak depends on the etiology, dimensions, and site. A small leak may resolve; however, a significant leak may require surgical intervention. Conservative management and applying a lumbar epidural blood patch may be advised if the leak continues. A lumbar epidural blood patch involves injecting a tiny volume of autologous blood into the vicinity of the spinal cord at the area of leakage. This facilitates the sealing of the leak and mitigates the symptoms related to it. Alternative treatments for a cerebrospinal fluid leak may include endoscopic repair and surgical repair.
Conservative management of a cerebrospinal fluid leak may include bed rest, which alleviates pressure on the afflicted region and facilitates healing of the leak. Pain management: Non-prescription analgesics like ibuprofen or acetaminophen may be advised to alleviate headaches and cervical discomfort. Hydration: Consuming enough water aids in sustaining sufficient bodily fluid levels and alleviating symptoms. Limitation of caffeine: Caffeine may exacerbate symptoms and should be avoided or restricted. Modifications in position: Refraining from actions that elevate pressure on the impacted region, such as sneezing, coughing, or straining, may alleviate discomfort. Acetazolamide administered at a dose of 500 mg 12 hourly for the first week, then reduced to 250 mg twice a day for the following week, seems to achieve a 100% success rate in the closure of the main defect in spontaneous cerebrospinal fluid leaks with regulated intracranial pressure. [89] Experts assert that acetazolamide must be supplied to individuals displaying spontaneous cerebrospinal fluid leaks and indications of high CSF pressure. Acetazolamide decreases CSF production by 48%. [90]
Conservative therapy is a choice for mild cases of CSF leaks and may be beneficial in allowing the leak to heal on its own. Nevertheless, more intrusive interventions, such as surgical correction, may be necessary in severe cases or if symptoms persist. Collaborating closely with a proficient healthcare practitioner is essential for determining the appropriate course of action, which should be individualized accordingly.
The epidural blood patch (EBP) method is a frequently used intervention for CSF leakage. The technique is to inject a tiny volume of the patient's autologous blood into the epidural space around the spinal cord to occlude the leak and avert further cerebrospinal fluid loss. [91] The EBP technique is often conducted under local anesthesia, with the patient positioned prone. The anesthesiologist anesthetizes the skin and tissues of the lower back and subsequently uses a needle to introduce a catheter into the epidural area. The patient's autologous blood (between 10 and 55 ml) is fed gradually via the catheter, occupying the epidural space and exerting pressure on the leakage site to facilitate closure. The EBP method is generally regarded as a safe and efficacious therapy for CSF leaks, with a high success rate for alleviating symptoms. It is often used in individuals not treated with conventional therapy or who have encountered a recurring leak. No significant difference was observed in the success rates of target and non-target patching procedures. [92]
The EBP method is essential for neurosurgeons and other professionals in addressing CSF leakage. The treatment is straightforward, minimally invasive, and may rapidly alleviate symptoms in most cases. Success rates for EBP have risen with the quantity of autologous blood, reaching 80% with 10 to 15 ml and exceeding 95% with 20 ml. [93]
The lumbar drain operation involves the insertion of a catheter into the lumbar portion of the spinal canal, where cerebrospinal fluid is collected. The lumbar drain procedure often provides temporary symptom relief and allows the cerebrospinal fluid leak to heal. The dimensions and position of the leak, together with the patient's healing trajectory, dictate the duration for which the catheter must be retained. A small incision was made in the lower back, and a slender tube was inserted. The cerebrospinal fluid can then be extracted from the body by connecting the tube to a drainage system. The patient often remains hospitalized for observation and necessary follow-up treatment for several days after the procedure. The procedure is often performed under local or general anesthesia; however, local anesthesia is enough because of its low risk. [94]
Surgery is advised when the leak source is located, symptoms remain unresponsive to less-invasive therapies, and severe symptoms continue to manifest. The surgeon locates the leak site and employs specialized devices to apply absorbent materials, such as Gelfoam and/or fibrin glue, to seal the breach in the nasal passage.
Limited data indicate that most patients with cranial CSF leakage demonstrate improvement with treatment. The resolution of the leak and associated symptoms appears to be more probable in patients with posttraumatic or postoperative CSF leaks and an identified dural defect than in those with a spontaneous CSF leak. [95]
This review has limitations, including a narrow scope of literature, trial secrecy, varying quality of studies, publication bias, lack of standardization, temporal factors, and authors' biases. These factors may lead to an incomplete understanding of complications, underestimating risks, and influencing conclusions. Addressing these limitations in future research could improve patient safety and enhance understanding of LP complications.
Lumbar puncture is a commonly used technique that yields significant diagnostic insights into central nervous system disorders. The related risks, such as post-LP meningitis, hemorrhagic complications, and grave neurological consequences, emphasize the need for increased vigilance and a meticulous approach. The literature analysis underscores the need for further study to enhance understanding of these problems and to develop appropriate therapies. Enhanced reporting and research methodologies might strengthen safety standards and optimize patient outcomes, ensuring that the advantages of lumbar puncture surpass the dangers in practical practice.
ACKNOWLEGMENT
The corresponding author and other authors acknowledge the support of the Open Libyan University.
AUTHORS' CONTRIBUTION
All authors have significantly contributed to the work, whether by conducting literature searches, drafting, revising, or critically reviewing the article. They have given their final approval of the version to be published, have agreed with the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
SOURCE OF FUNDING
None.
CONFLICT OF INTEREST
None.
References
- Frederiks J, Koehler P. The first lumbar puncture. Journal of the History of the Neurosciences. 1997;6(2):147-53.
- Doherty CM, Forbes RB. Diagnostic lumbar puncture. The Ulster medical journal. 2014;83(2):93.
- Wright BL, Lai JT, Sinclair AJ. Cerebrospinal fluid and lumbar puncture: a practical review. Journal of neurology. 2012;259:1530-45.
- Sternbach G. Lumbar puncture. The Journal of emergency medicine. 1985;2(3):199-203.
- Armon C, Evans RW. Addendum to assessment: Prevention of post-lumbar puncture headaches: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65(4):510-2.
- Jane LA, Wray AA. Lumbar puncture. StatPearls [Internet]: StatPearls Publishing; 2023.
- Dobrocky T, Nicholson P, Häni L, Mordasini P, Krings T, Brinjikji W, et al. Spontaneous intracranial hypotension: searching for the CSF leak. The Lancet Neurology. 2022;21(4):369-80.
- D'Antona L, Jaime Merchan MA, Vassiliou A, Watkins LD, Davagnanam I, Toma AK, et al. Clinical Presentation, Investigation Findings, and Treatment Outcomes of Spontaneous Intracranial Hypotension Syndrome: A Systematic Review and Meta-analysis. JAMA Neurol. 2021;78(3):329-37.
- Severson M, Schaurich CG, Strecker-McGraw MK. Cerebrospinal Fluid Leak. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024.
- Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128(6):309-16.
- Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda). 2010;25(4):239-49.
- Owler BK, Pitham T, Wang D. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res. 2010;7:15.
- Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21(3-4):79-96.
- Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol. 2015;273:57-68.
- Gorelick PB, Biller J. Lumbar puncture. Technique, indications, and complications. Postgrad Med. 1986;79(8):257-68.
- Hasbun R. Infections of the central nervous system. Pathy's Principles and Practice of Geriatric Medicine2022. p. 1305-25.
- Fautsch KJ, Block DR, Graff-Radford J, Wang F, Craver EC, Hodge DO, et al. Population-Based Evaluation of Total Protein in Cerebrospinal Fluid. Mayo Clin Proc. 2023;98(2):239-51.
- Hegen H, Auer M, Deisenhammer F. Serum glucose adjusted cut-off values for normal cerebrospinal fluid/serum glucose ratio: implications for clinical practice. Clin Chem Lab Med. 2014;52(9):1335-40.
- Tan QC, Xing XW, Zhang JT, He MW, Ma YB, Wu L, et al. Correlation between blood glucose and cerebrospinal fluid glucose levels in patients with differences in glucose metabolism. Front Neurol. 2023;14:1103026.
- Nigrovic LE, Kimia AA, Shah SS, Neuman MI. Relationship between cerebrospinal fluid glucose and serum glucose. N Engl J Med. 2012;366(6):576-8.
- Leen WG, Willemsen MA, Wevers RA, Verbeek MM. Cerebrospinal fluid glucose and lactate: age-specific reference values and implications for clinical practice. PLoS One. 2012;7(8):e42745.
- Rahimi J, Woehrer A. Overview of cerebrospinal fluid cytology. Handb Clin Neurol. 2017;145:563-71.
- Breuer AC, Tyler HR, Marzewski DJ, Rosenthal DS. Radicular vessels are the most probable source of needle-induced blood in lumbar puncture: significance for the thrombocytopenic cancer patient. Cancer. 1982;49(10):2168-72.
- Edlow JA, Bruner KS, Horowitz GL. Xanthochromia. Arch Pathol Lab Med. 2002;126(4):413-5.
- Chu K, Hann A, Greenslade J, Williams J, Brown A. Spectrophotometry or visual inspection to most reliably detect xanthochromia in subarachnoid hemorrhage: systematic review. Ann Emerg Med. 2014;64(3):256-64.e5.
- The Diagnostic Spinal Tap. Annals of Internal Medicine. 1986;104(6):880-5.
- Kim KT. Lumbar puncture: considerations, procedure, and complications. Encephalitis. 2022;2(4):93-7.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345(24):1727-33.
- Engelborghs S, Niemantsverdriet E, Struyfs H, Blennow K, Brouns R, Comabella M, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement (Amst). 2017;8:111-26.
- Duits FH, Martinez-Lage P, Paquet C, Engelborghs S, Lleó A, Hausner L, et al. Performance and complications of lumbar puncture in memory clinics: Results of the multicenter lumbar puncture feasibility study. Alzheimers Dement. 2016;12(2):154-63.
- Fernandez M, Moylett EH, Noyola DE, Baker CJ. Candidal meningitis in neonates: a 10-year review. Clinical infectious diseases. 2000;31(2):458-63.
- Saraya AW, Wacharapluesadee S, Petcharat S, Sittidetboripat N, Ghai S, Wilde H, et al. Normocellular CSF in herpes simplex encephalitis. BMC research notes. 2016;9:1-7.
- Soares CN, Cabral-Castro MJ, Peralta JM, de Freitas MR, Zalis M, Puccioni-Sohler M. Review of the etiologies of viral meningitis and encephalitis in a dengue endemic region. Journal of the neurological sciences. 2011;303(1-2):75-9.
- Chen J-H, Wu J, Yang X-Y, Li J, Huang N-Q, Shi S-P, et al. Diagnostic value of the Electroencephalogram and Cerebrospinal Fluid in viral encephalitis. The neurologist. 2022;27(5):225-9.
- Nizam A, Sivakumar K, Yacoub H. Froin Syndrome, a Rare Complication of Multiple Myeloma. The Neurologist. 2021;26(3):83-5.
- Garispe A, Naji H, Dong F, Arabian S, Neeki M. Froin's syndrome secondary to traumatic and infectious etiology. Cureus. 2019;11(12).
- Mantese CE, Lubini R. Froin's syndrome with tuberculosis myelitis and spinal block. Revista da Associação Médica Brasileira. 2022;68:10-2.
- Langenbruch L, Wiendl H, Groß C, Kovac S. Diagnostic utility of cerebrospinal fluid (CSF) findings in seizures and epilepsy with and without autoimmune-associated disease. Seizure. 2021;91:233-43.
- Süße M, Gag K, Hamann L, Hannich M, von Podewils F. Time dependency of CSF cell count, lactate and blood-CSF barrier dysfunction after epileptic seizures and status epilepticus. Seizure. 2022;95:11-6.
- Bueno CC, Llovet MA, Ruiz MM, Mardones PM, Gomez FG, Santiago RT. Hypoglycorrhachia in mumps meningitis (author's transl). Anales espanoles de pediatria. 1978;11(8-9):547-52.
- Zisimopoulou V, Mamali M, Katsavos S, Siatouni A, Tavernarakis A, Gatzonis S. Cerebrospinal fluid analysis after unprovoked first seizure. Functional Neurology. 2016;31(2):101.
- Bihan K, Weiss N, Théophile H, Funck‐Brentano C, Lebrun‐Vignes B. Drug‐induced aseptic meningitis: 329 cases from the French pharmacovigilance database analysis. British Journal of Clinical Pharmacology. 2019;85(11):2540-6.
- Auriel E, Regev K, Korczyn AD. Nonsteroidal anti-inflammatory drugs exposure and the central nervous system. Handbook of clinical neurology. 2014;119:577-84.
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole–induced aseptic meningitis—not just another sulfa allergy. Annals of Allergy, Asthma & Immunology. 2014;113(5):520-6.
- Agabawi S. Trimethoprim‐Sulfamethoxazole‐Induced Aseptic Meningitis: A Rare Presentation of Commonly Used Antibiotic. Case Reports in Infectious Diseases. 2019;2019(1):4289502.
- Bechard P, Perron G, Larochelle D, Lacroix M, Labourdette A, Dolbec P. Case report: epidural blood patch in the treatment of abducens palsy after a dural puncture. Canadian Journal of Anesthesia. 2007;54(2):146.
- Anwar S, Nalla S, Fernando DJ. Abducens nerve palsy as a complication of lumbar puncture. European Journal of Internal Medicine. 2008;19(8):636-7.
- Follens I, Godts D, Evens P, Tassignon M. Combined fourth and sixth cranial nerve palsy after lumbar puncture: a rare complication. A case report. BULLETIN-SOCIETE BELGE D OPHTALMOLOGIE. 2001:29-34.
- del-Rio–Vellosillo M, Garcia-Medina JJ, Pinazo-Duran MD, Abengochea-Cotaina A, Barbera-Alacreu M. Ocular motor palsy after spinal puncture. Regional Anesthesia & Pain Medicine. 2017;42(1):1-9.
- Warltier DC, Sprung J, Bourke DL, Contreras MG, Warner ME, Findlay J. Perioperative hearing impairment. The Journal of the American Society of Anesthesiologists. 2003;98(1):241-57.
- Finegold H, Mandell G, Vallejo M, Ramanathan S. Does spinal anesthesia cause hearing loss in the obstetric population? Anesthesia & Analgesia. 2002;95(1):198-203.
- Fog J, Wang LP, Sundberg A, Mucchiano C. Hearing loss after spinal anesthesia is related to needle size. Anesthesia & Analgesia. 1990;70(5):517-22.
- Erol A, Topal A, Arbag H, Kilicaslan A, Reisli R, Otelcioglu S. Auditory function after spinal anaesthesia: the effect of differently designed spinal needles. European Journal of Anaesthesiology| EJA. 2009;26(5):416-20.
- Narchi P, Veyrac P, Viale M, Benhamou D. Long-term postdural puncture auditory symptoms: effective relief after epidural blood patch. Anesthesia & Analgesia. 1996;82(6):1303.
- Epstein NE. Neurological complications of lumbar and cervical dural punctures with a focus on epidural injections. Surgical Neurology International. 2017;8.
- Moore AR, Wieczorek PM, Carvalho JC. Association between post–dural puncture headache after neuraxial anesthesia in childbirth and intracranial subdural hematoma. JAMA neurology. 2020;77(1):65-72.
- Guglielminotti J, Landau R, Li G. Major neurologic complications associated with postdural puncture headache in obstetrics: a retrospective cohort study. Anesthesia & Analgesia. 2019;129(5):1328-36.
- Paun T, Zavoreo I, Jurašić M-J, Jadrijević Tomas A, Bašić Kes V. Spinal Subdural Hematoma Associated with Lumbar Puncture–a Case Report. Acta clinica Croatica. 2022;61(1.):149-51.
- Brown MW, Yilmaz TS, Kasper EM. Iatrogenic spinal hematoma as a complication of lumbar puncture: What is the risk and best management plan? Surgical neurology international. 2016;7(Suppl 22):S581.
- Edwards LS, Cuganesan R, Cappelen-Smith C. Cerebral venous thrombosis as a complication of intracranial hypotension after lumbar puncture. BMJ Neurology Open. 2020;2(2).
- Honig A, Eliahou R, Pikkel Y, Leker RR. Iatrogenic intracranial hypotension and cerebral venous thrombosis. Journal of the Neurological Sciences. 2016;366:191-4.
- Schievink WI, Maya M, Prasad RS, Wadhwa VS, Cruz RB, Moser FG. Spinal CSF-Venous Fistulas in Morbidly and Super Obese Patients with Spontaneous Intracranial Hypotension. AJNR Am J Neuroradiol. 2021;42(2):397-401.
- Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond). 2013;37(6):889-91.
- Chazen JL, Robbins MS, Strauss SB, Schweitzer AD, Greenfield JP. MR Myelography for the Detection of CSF-Venous Fistulas. AJNR Am J Neuroradiol. 2020;41(5):938-40.
- Hsieh CJ, Kuo LT, Lai DM, Huang AP. Cervical cerebrospinal fluid venous fistula with syringomyelia treated with suboccipital decompression: illustrative case. J Neurosurg Case Lessons. 2022;3(15).
- Korein J, Cravioto H, Leicach M. Reevaluation of lumbar puncture: a study of 129 patients with papilledema or intracranial hypertension. Neurology. 1959;9(4):290-.
- Duffy G. Lumbar puncture in spontaneous subarachnoid haemorrhage. Br Med J (Clin Res Ed). 1982;285(6349):1163-4.
- Costerus JM, Brouwer MC, Sprengers ME, Roosendaal SD, van der Ende A, van de Beek D. Cranial computed tomography, lumbar puncture, and clinical deterioration in bacterial meningitis: a nationwide cohort study. Clinical Infectious Diseases. 2018;67(6):920-6.
- Chik Y, Hoesch RE, Lazaridis C, Weisman CJ, Llinas RH. A case of postpartum cerebral angiopathy with subarachnoid hemorrhage. Nature Reviews Neurology. 2009;5(9):512-6.
- Minai FN, Hasan SF, Sheerani M. Post-dural puncture posterior reversible encephalopathy syndrome. Journal of the College of Physicians and Surgeons Pakistan. 2011;21(1):37.
- Doherty H, Hameed S, Ahmed I, Russell I. Post-dural puncture headache and posterior reversible encephalopathy syndrome: a misdiagnosis or co-presentation? International Journal of Obstetric Anesthesia. 2014;23(3):279-82.
- Lee S-J, Lin Y-Y, Hsu C-W, Chu S-J, Tsai S-H. Intraventricular hematoma, subarachnoid hematoma and spinal epidural hematoma caused by lumbar puncture: an unusual complication. The American journal of the medical sciences. 2009;337(2):143-5.
- Evans RW. Complications of lumbar puncture. Neurologic clinics. 1998;16(1):83-105.
- Hasegawa K, Yamamoto N. Nerve root herniation secondary to lumbar puncture in the patient with lumbar canal stenosis: A case report. Spine. 1999;24(9):915-7.
- Mims SC, Tan HS, Sun K, Pham T, Rubright S, Kaplan SJ, et al. Long-term morbidities following unintentional dural puncture in obstetric patients: a systematic review and meta-analysis. Journal of Clinical Anesthesia. 2022;79:110787.
- Miyake S, Kobayashi N, Murai N, Kondoh T, Kohmura E. Acquired Lumbar Epidermoid Cyst in an Adult—Case Report—. Neurologia medico-chirurgica. 2005;45(5):277-9.
- Prat Acín R, Galeano I. Giant occipital intradiploic epidermoid cyst associated with iatrogenic puncture. Acta neurochirurgica. 2008;150:413-4.
- McDonald JV, Klump TE. Intraspinal epidermoid tumors caused by lumbar puncture. Archives of neurology. 1986;43(9):936-9.
- Bakhsh A, Elmolla M, Buxton N, Brodbelt A. Chronic CSF leak from lumbar-peritoneal shunt tract: A case report. Surgical Neurology International. 2022;13.
- Tang R, Mao S, Li D, Ye H, Zhang W. Treatment and outcomes of iatrogenic cerebrospinal fluid leak caused by different surgical procedures. World Neurosurgery. 2020;143:e667-e75.
- Le C, Strong EB, Luu Q. Management of anterior skull base cerebrospinal fluid leaks. Journal of Neurological Surgery Part B: Skull Base. 2016:404-11.
- D’Antona L, Merchan MAJ, Vassiliou A, Watkins LD, Davagnanam I, Toma AK, et al. Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome: a systematic review and meta-analysis. JAMA neurology. 2021;78(3):329-37.
- Strupp M, Katsarava Z. Postpunktionelles und spontanes Liquorunterdrucksyndrom: Post-lumbar puncture syndrome and spontaneous low CSF pressure syndrome. Der Nervenarzt. 2009;80:1509-19.
- Farnsworth PJ, Madhavan AA, Verdoorn JT, Shlapak DP, Johnson DR, Cutsforth-Gregory JK, et al. Spontaneous intracranial hypotension: updates from diagnosis to treatment. Neuroradiology. 2023;65(2):233-43.
- Nandapalan V, Watson ID, Swift AC. Beta-2-transferrin and cerebrospinal fluid rhinorrhoea. Clin Otolaryngol Allied Sci. 1996;21(3):259-64.
- McCudden CR, Senior BA, Hainsworth S, Oliveira W, Silverman LM, Bruns DE, et al. Evaluation of high resolution gel β(2)-transferrin for detection of cerebrospinal fluid leak. Clin Chem Lab Med. 2013;51(2):311-5.
- Meco C, Oberascher G. Comprehensive algorithm for skull base dural lesion and cerebrospinal fluid fistula diagnosis. Laryngoscope. 2004;114(6):991-9.
- Oakley GM, Orlandi RR, Woodworth BA, Batra PS, Alt JA. Management of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6(1):17-24.
- Ramakrishnan N, Roy R, Singh S, Goyal S, Gupta D, Chugh R. Approach to Management of Cerebrospinal Fluid Rhinorrhea: Institutional Based Protocol. Indian Journal of Otolaryngology and Head & Neck Surgery. 2022:1-8.
- Wang EW, Vandergrift WA, 3rd, Schlosser RJ. Spontaneous CSF Leaks. Otolaryngol Clin North Am. 2011;44(4):845-56, vii.
- Choi SY, Seong M, Kim EY, Youn MS, Cho S, Jang H, et al. Outcome of epidural blood patch for imaging-negative spontaneous intracranial hypotension. Cephalalgia. 2023;43(2):03331024221140471.
- Signorelli F, Caccavella VM, Giordano M, Ioannoni E, Caricato A, Polli FM, et al. A systematic review and meta-analysis of factors affecting the outcome of the epidural blood patching in spontaneous intracranial hypotension. Neurosurgical Review. 2021;44(6):3079-85.
- Smith KA. Spontaneous intracranial hypotension: Targeted or blind blood patch. Journal of Clinical Neuroscience. 2016;25:10-2.
- Khan R, Sajjad M, Khan AA, Ahmad B, Ahmad S, Mushtaq M, et al. Comparison Of Lumbar Drain Insertion And Conservative Management In The Treatment Of Traumatic CSF Rhinorrhoea. Journal of Ayub Medical College Abbottabad-Pakistan. 2019;31(3).
- 0Stevens SM, Smith CJ, Lawton M. Postoperative management of patients with spontaneous cerebrospinal fluid leak. Curr Opin Otolaryngol Head Neck Surg. 2019;27(5):361-8.
