Full HTML
Association of vitamin D deficiency with dyslipidemia, glycemic control, and microalbuminuria in patients with Type 2 diabetes mellitus in Qatar
Gowri Karuppasamy1, Shaikha Al Shokri2, Aseel Sukik3, Mohamed Elmudathir Osman4
Author Affiliation
1Consultant, Department of Medicine, Hamad General Hospital,
2 Senior Consultant, Department of Endocrinology, Hamad Medical Corporation, Doha, Qatar
Abstract
Background: Emerging data found that Type 2 diabetes mellitus (T2DM) is associated with Vitamin D deficiency at various frequencies. This study aims to estimate the prevalence of Vitamin D deficiency in T2DM patients in Qatar and the correlation between Vitamin D deficiency and other variables such as dyslipidemia, glycemic control, and microalbuminuria. Methods: This retrospective cross-sectional analytical study was conducted in the medical outpatient clinic at Hamad General Hospital. The study involved adult patients (≥18 years) with T2DM. The study covered patients admitted between January 1, 2018, and July 31, 2018. Ethical approval was obtained from the Medical Research Committee. Results: We recruited 400 subjects with T2DM. Their mean age was 58.97±10.32 years, and the majority were women (52.0%) and Arabs (69.5%). The mean duration of Type 2 diabetes diagnosis was 14.94±8.99 years. The prevalence of Vitamin D deficiency was 29.5%. A comparison between Vitamin D deficiency and non-vitamin D deficiency groups showed a statistically significant difference in terms of fasting blood (FB) sugar (p<0.001), random blood (RB) sugar (p<0.001), hemoglobin A1c (HBA1c) (p<0.001), total cholesterol (P = 0.001), low-density lipoprotein cholesterol (LDL [C]) (p=0.004), high-density lipoprotein cholesterol (HDL [C]) (p<0.001), triglyceride (p<0.001), and urinary albumin excretion rate (UAER) (p=0.007). Data analysis showed that a significant negative correlation was found between Vitamin D level and FB sugar (r=−0.208, p<0.001), RB sugar (r=−0.20, p<0.001), HBA1c (r=−0.260, p<0.001), total cholesterol (r=−0.218, p<0.001), LDL (C) (r=−0.176, p=0.004), triglyceride (r=−0.342, p<0.001), and UAER (r=−0.184, p=0.007). Conclusion: Our study showed a significant correlation between Vitamin D deficiency and the poor control of T2DM, dyslipidemia, and microalbuminuria. The results emphasize the importance of monitoring Vitamin D status in high-risk populations.
DOI: 10.32677/yjm.v1i1.3292
Keywords: Dyslipidemia, Glycemic control, Microalbuminuria, Type 2 diabetes mellitus, Vitamin D
Pages: 17-21
View: 3
Download: 13
DOI URL: https://doi.org/10.32677/yjm.v1i1.3292
Publish Date: 25-03-2025
Full Text
Type 2 diabetes mellitus (T2DM) is a serious and growing global public health problem with a significant economic burden. Approximately 463 million people are living with diabetes in 2019, and the number is expected to reach 700 million by 2045. A recent modeling study estimates that the prevalence of T2DM among Qataris will rise from 17% in 2012 to at least 24% by 2050 in the absence of urgent and sufficient action [1,2]. Chronic complications, such as chronic kidney diseases and cardiovascular diseases, are well-known outcomes of T2DM progression that reduces patients’ quality of life and increases the burden on the health-care system and diabetes mortality. As a result, it is essential to identify the main modifiable factors related to these complications to improve the prognosis of T2DM.
Vitamin D is an important hormone for calcium and phosphorus homeostasis and bone. It was found that T2DM is associated with Vitamin D deficiency at various frequencies. Recently, Vitamin D has acquired a large interest as a possible factor in the pathogenesis and prevention of diabetes. In a systematic review, it was concluded that Vitamin D and calcium deficiency can have a deleterious impact on glycemia, while glucose metabolism can be improved by a combination supplementation with both nutrients [3]. Other systematic review articles suggested the possible role of Vitamin D in the protection against some musculoskeletal disorders, infectious diseases, autoimmune diseases, cardiovascular disease, Type 1 and T2DM, several types of cancer, mental illness, infertility, and adverse pregnancy and birth outcomes [4-6]. Recent trial results are consistent with a large body of evidence from observational studies indicating that Vitamin D plays a role in modulating diabetes risk [7]. However, the causal relationship between Vitamin D deficiency and T2DM
has not been established by randomized clinical trials, and, thus, will remain a matter of debate. In Qatar, no previous studies examined the association between Vitamin D and T2DM in adult patients. Therefore, we designed this study to estimate the prevalence of Vitamin D deficiency in T2DM patients in Qatar and assess the correlation between Vitamin D deficiency and other variables such as dyslipidemia, glycemic control, and microalbuminuria. MATERIALS AND METHODS Study Design, Population, and Setting This retrospective cross-sectional analytical study was conducted in the medical outpatient clinic at Hamad General Hospital. The study involved adult patients (≥18 years) with T2DM, between January 1, 2018, and July 31, 2018. Ethical approval was obtained from the Medical Research Committee (proposal number # MRC-01-18-289). Inclusion and Exclusion Criteria We excluded patients with other types of diabetes, namely, Type 1 diabetes, gestational diabetes mellitus, diabetes due to genetic abnormalities of beta-cell function or insulin action, and drug-induced diabetes. We also excluded pregnant and lactating women, patients taking Vitamin D (D2 and D3 supplements) and calcium supplements, patients with malabsorption owed to bowel disease or post-bariatric surgery, patients taking medications such as anticonvulsants, HIVAIDS therapy, steroids, rifampin, cholestyramine, or orlistat. On the other hand, adult patients (≥18 years) with T2DM who were using the daily anti-diabetic treatment and statin therapy for at least 3 months and who did not meet the above criteria were recruited into this study
Definitions and Diagnostic Criteria
1. Vitamin D status was classified as follows: Serum Vitamin D level 30–50 ng/mL was considered as Vitamin D sufficiency (Optimum) [8]. Since there was no consensus on the level of Vitamin D that denotes insufficiency and to avoid confusion or misunderstanding on the part of readers, we have used the term Vitamin D deficiency throughout the text to refer to patients with Vitamin D levels 100–200), severe (>200–300), and macroalbuminuria (>300) g/mg. The term microalbuminuria was used to describe urinary albumin excretion within 30–300 g/mg creatinine [8] 3. Hemoglobin A1c (HbA1c) ≤ 8% was considered as controlled levels and >8% as uncontrolled levels [9] 4. Estimated glomerular filtration rate values (eGFR) were calculated according to the chronic kidney disease epidemiology collaboration formula [10].
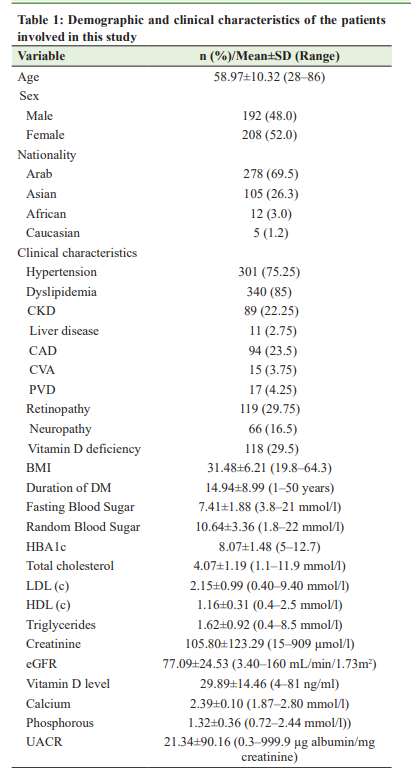
Sample Size A sample size of 400 subjects was obtained, which provided a power of 80% at an alpha value of 0.05. Data Analysis Descriptive statistics of qualitative and quantitative data were expressed in the form of frequency along with percentage and mean±Standard Deviation (SD). An independent t-test was used to compare the difference in the means of blood glucose, HbA1c, lipids, and UAER between diabetic patients with and without a Vitamin D deficiency. Pearson correlation analyzes were performed to examine the linear relationship between Vitamin D deficiency and dyslipidemia, glycemic control, and microalbuminuria. Data analysis was performed with SPSS software (v 23; IBM Corp, Armonk, NY, USA). RESULTS Demographic and Clinical Data We recruited 400 subjects with T2DM. Their mean age was 58.97±10.32 years (range of 28–86 years) and the majority were women (52.0%) and Arabs (69.5%). The mean duration of Type 2 diabetes diagnosis was 14.94±8.99 years (range of 1–50 years) and mean BMI was 31.48±6.21 kg/m2 (range of 19.8–64.3 kg/m2). Table 1 describes the demographic and clinical characteristics of the study population. The prevalence of Vitamin D deficiency was 29.5%.
Comparison between Vitamin D Deficiency and non-vitamin D deficiency groups
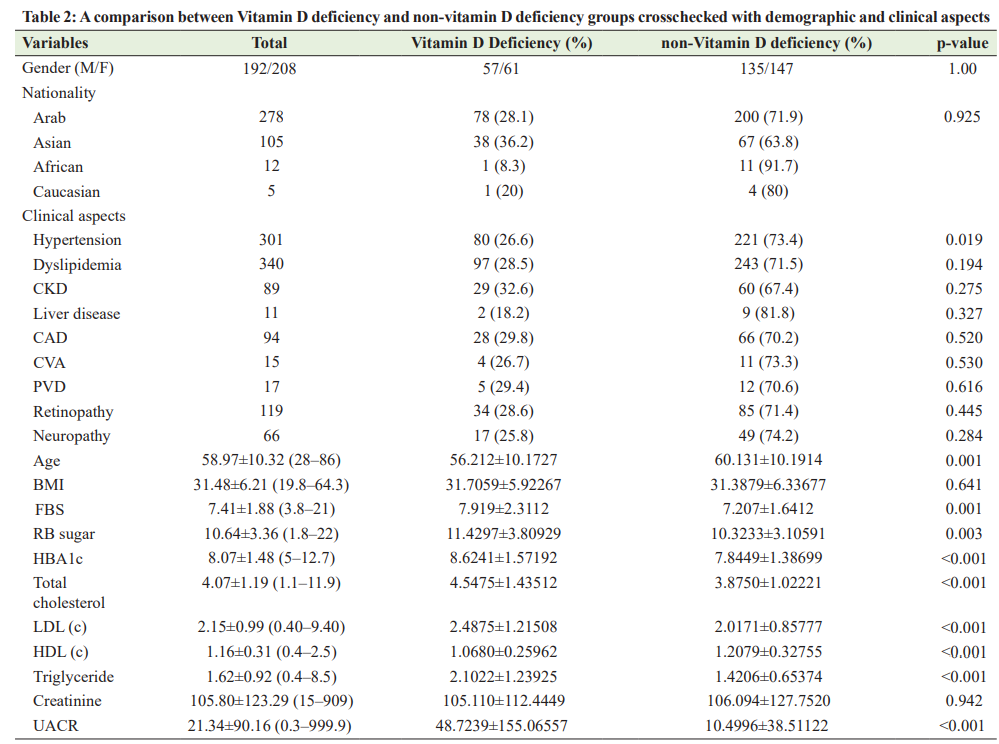
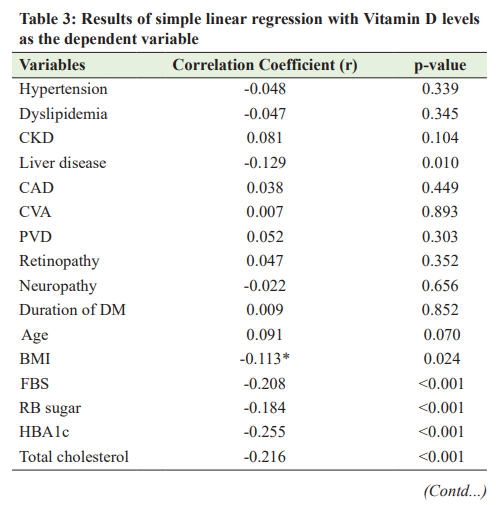
A comparison between Vitamin D deficiency and non-vitamin D deficiency groups showed a statistically significant difference in terms of fasting blood (FB) sugar (p<0.001), random blood (RB) sugar (p<0.001), HBA1c (p<0.001), total cholesterol (p=0.001), low-density lipoprotein cholesterol (LDL [C]) (p=0.004), highdensity lipoprotein cholesterol (HDL [C]) (p<0.001), triglyceride (p<0.001), and UACR (p=0.007). Table 2 summarizes the comparison between Vitamin D deficiency and non-vitamin D deficiency groups in relation to demographic and clinical aspects. Correlation between Vitamin D Deficiency and Dyslipidemia and Glycemic Control and Microalbuminuria The correlations between the level of serum Vitamin D and FB sugar, RB sugar, glycated hemoglobin (HBA1c), total cholesterol, LDL (C), HDL (C), triglyceride, and UACR were described in Table 2. Data analysis showed that a significant negative correlation was found between Vitamin D level and FB sugar (r=−0.208, p<0.001), RB sugar (r=−0.20, p<0.001), HBA1c
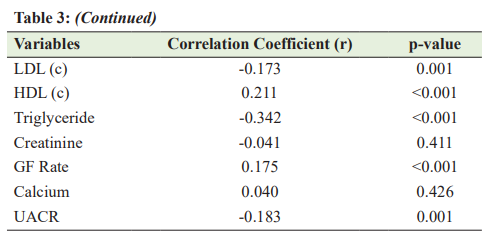
(r=−0.260, p<0.001), total cholesterol (r=−0.218, p<0.001), LDL (C) (r=−0.176, p=0.004), triglyceride (r=−0.342, p<0.001), and UACR (r=−0.184, p=0.007). Table 3 describes the correlation between Vitamin D level and other variables. DISCUSSION Recently, researchers began to show increased interest in the association between Vitamin D deficiency and diabetes mellitus. To the best of our knowledge, this is the first study designed to assess the association between Vitamin D and T2DM in adult patients living in Qatar. The effect of Vitamin D on -cell function and insulin sensitivity has been observed in animal and human studies. Vitamin D improves insulin production and sensitivity. It facilitates the biosynthetic capacity of cells and, also, accelerates the conversion of proinsulin into insulin [11-13]. Emerging evidence suggests that Vitamin D is involved in the etiology and pathogenesis of diabetes mellitus [14], leading to the hypothesis that Vitamin D insufficiency is positively correlated with insulin resistance and cardiovascular risk in obese adolescents. This also shows that Vitamin D supplementation improves insulin resistance and cardiovascular risk factors in this population [15]. The reported prevalence of Vitamin D deficiency among patients with T2DM ranges from 62% to 91% [16,17]. In our study, the prevalence of Vitamin D deficiency was 29.5%. The low prevalence was not true, as we involved only the patients with Vitamin D levels of <20 ng/ml. In general, our findings are in line with most of the recent studies. In the present study, the bivariate analysis showed that FB sugar, RB sugar, and HBA1c were affected and uncontrolled in the Vitamin D deficiency group compared with the non-non-Vitamin D deficiency Group, in line with the results observed by Anyanwu et al. [18]. Moreover, a significant inverse correlation between glycemic control and Vitamin D levels has been reported by several authors [17-19] This provides further evidence that low serum Vitamin D can play a significant role in impaired glucose metabolism. However, the hypothesis that Vitamin D supplementation may improve insulin resistance and cardiovascular risk factors in this population has not yet been proven, and prescribing supplemental Vitamin D during the early phase of diabetes is still experimental [20]. There were other findings in this study, including the association between Vitamin D deficiency and dyslipidemia. We found significantly increased mean total cholesterol, LDL (C), and triglyceride levels in subjects with Vitamin D deficiency compared with those with the non-Vitamin D deficiency Group. This finding has been reported by several authors [17,21-23]. The functions of Vitamin D are linked to lipid values in several ways: First, Vitamin D regulates calcium metabolism and increases intestinal calcium absorption, thereby reducing intestinal fatty acid absorption [24]. Therefore, a reduction in intestinal fat absorption can lower the cholesterol level. In addition, increasing the calcium concentration promotes the conversion of cholesterol into bile acids in the liver, resulting in reduced cholesterol levels [25]. It was also found that there was an increased frequency of microalbumin in the Vitamin D deficient group compared to the non-Vitamin D deficiency Group. In addition, we also found that Vitamin D level was inversely correlated with microalbumin levels, which agrees with the results of Balla et al. and Fiscella et al. [26,27]. This suggests that studies to further characterize the role of Vitamin D as a possible risk factor in diabetic nephropathy are needed to assess the impact of maintaining adequate Vitamin D levels on the progression of diabetic nephropathy, as some studies have shown that Vitamin D replacement therapy reduces albuminuria in patients with chronic kidney disease [27,28]. On
the other hand, some studies have provided conflicting results regarding the association between Vitamin D status and diabetic nephropathy [29-31]. There are limitations to this study that should be considered: First, it was a retrospective analysis and, therefore, relied on secondary data. Second, we involved only patients with Vitamin D levels of <20 ng/l because there was no consensus on the level of Vitamin D that denotes insufficiency, so there is a possibility that we lost patients in the prevalence estimation. Third, because this was a hospital-based study, the results cannot be generalized. Despite these limitations, this study is the first to highlight the relationship between Vitamin D deficiency and glycemic control, dyslipidemia, and microalbuminuria in Qatar.
CONCLUSION Our study showed a significant relationship between Vitamin D deficiency and glycemic control, dyslipidemia, and microalbuminuria. The results emphasize the need for better awareness among researchers and clinicians about the consequences of Vitamin D deficiency and the importance of monitoring its status in high-risk populations. Increasing population awareness is also essential to overcome Vitamin D deficiency in the population of Qatar. However, these aspects are worth investigating with large prospective studies and with adequate follow-up.
AUTHORS’ CONTRIBUTION Karuppasamy G wrote the proposal, analyzed the data, and wrote the final manuscript. Al Shokri S proposed the idea, reviewed the literature, and aided in the data collection. Sukik A aided in the data collection, research proposal writing, and data entry. Saleh AO aided in the data collection and data entry. Osman ME aided in research proposal writing, the data analysis, and the revision of the final manuscript. All authors read the manuscript and agree to its publication.
References
1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843.
2. Awad SF, O’Flaherty M, Critchley J, et al. Forecasting the burden of Type 2 diabetes mellitus in Qatar to 2050: A novel modeling approach. Diabetes Res Clin Pract 2018;137:100-8.
3. Pittas AG, Lau J, Hu FB, et al. The role of Vitamin D and calcium in Type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017-29.
4. Holick MF. Vitamin D: Extraskeletal health. Endocrinol Metab Clin North Am 2010;39:381-400.
5. Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev 2013;12:976-89.
6. Lips P, Eekhoff M, van Schoor N, et al. Vitamin D and Type 2 diabetes. J Steroid Biochem Mol Biol 2017;173:280-5.
7. Pittas AG, Jorde R, Kawahara T, et al. Vitamin D supplementation for prevention of Type 2 diabetes mellitus: To D or not to D? J Clin Endocrinol Metab 2020;105:3721-33.
8. Shaafie IA, Hesham RA, Basha AA. Vitamin D status in Type 2 diabetic patients and its association with glycemic control, lipids and microalbuminuria: A pilot study. GMJ ASM 2013;2:S6-13.
9. Aljack HA, Abdalla MK, Idris OF, et al. Vitamin D deficiency increases risk of nephropathy and cardiovascular diseases in Type 2 diabetes mellitus patients. J Res Med Sci 2019;24:47.
10. Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (chronic kidney disease epidemiology collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12.
11. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of Vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote Vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67:373-8.
12. Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol 2007;103:620-5.
13. Gannage-Yared MH, Chemali R, Yaacoub N, et al. Hypovitaminosis D in a sunny country: Relation to lifestyle and bone markers. J Bone Miner Res 2000;15:1856-62.
14. Michos ED. Vitamin D deficiency and the risk of incident Type 2 diabetes. Future Cardiol 2009;5:15-8.
15. Reis AF, Hauache OM, Velho G. Vitamin D endocrine system and the genetic susceptibility to diabetes, obesity and vascular disease. A review of evidence. Diabetes Metab 2005;31:318-25.
16. Bell NH, Greene A, Epstein S, et al. Evidence for alteration of Vitamin D-endocrine system in Blacks. J Clin Invest 1985;76:470-3.
17. Rolim MC, Santos BM, Conceição G, et al. Relationship between Vitamin D status, glycemic control and cardiovascular risk factors in Brazilians with Type 2 diabetes mellitus. Diabetol Metab Syndr 2016;8:77.
18. Anyanwu AC, Olopade OB, Onung SI, et al. Serum Vitamin D levels in persons with Type 2 diabetes mellitus in Lagos, Nigeria. Int J Diabetes Clin Res 2020;7:133.
19. Karau PB, Kirna B, Amayo E, et al. The prevalence of Vitamin D deficiency among patients with Type 2 diabetes seen at a referral hospital in Kenya. Pan Afr Med J 2019;34:38.
20. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral Vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: Randomised double blind controlled trial. BMJ 2003;326:469-75.
21. Karhapää P, Pihlajamäki J, Pörsti I, et al. Diverse associations of 25-hydroxyvitamin D and 1,25-dihydroxy-vitamin D with dyslipidaemias. J Intern Med 2010;268:604-61.
22. Auwerx J, Bouillon R, Kesteloot H. Relation between 25-hydroxyvitamin D3, apolipoprotein A-I, and high density lipoprotein cholesterol. Arteriosc Thromb 1992;12:671-4.
23. Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third national health and nutrition examination survey. Arch Intern Med 2007;167:1159-65.
24. Wang Y, Si S, Liu J, et al. The associations of serum lipids with Vitamin D status. PLoS One 2016;11:e0165157.
25. Vaskonen T, Mervaala E, Sumuvuori V, et al. Effects of calcium and plant sterols on serum lipids in obese Zucker rats on a low-fat diet. Br J Nutr 2007;87:239-45.
26. Balla DI, Abdalla AM, Elrayah ZA, et al. The association of 25(OH) Vitamin D level with glycemic control and nephropathy complication in Sudanese with Type 2 diabetes. Int J Med Res Health Sci 2018;7:62-8.
27. Fiscella KA, Winters PC, Ogedegbe G. Vitamin D and racial disparity in albuminuria: NHANES 2001-2006. Am J Hypertens 2011;24:1114-20.
28. Gembillo G, Cernaro V, Salvo A, et al. Role of Vitamin D status in diabetic patients with renal disease. Medicina (Kaunas) 2019;55:273.
29. de Boer IH, Sachs MC, Cleary PA, et a1. Circulating Vitamin D metabolites and kidney disease in Type 1 diabetes. J Clin Endocrinol Metab 2012;97:4780-8.
30. Joergensen C, Hovind P, Schmedes A, et a1. Vitamin D levels, microvascular complications, and mortality in Type l diabetes. Diabetes Care 2011;34:1081-5.
31. Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving HH, Rossing P, et al. Vitamin D levels and mortality in Type 2 diabetes. Diabetes Care 2010;33:2238-22.
