Full HTML
Application of chlorhexidine and its effect on hybrid layers: A review of the literature
Saimir Heta1, Ilma Robo2, Eva Haxhiu3, Blerta Rumano3, Vera Ostreni4, Edona Hasanaj5, Sonila Kapaj6
Author Affiliation
1Consultant, Pediatric Surgeon, Department of Pediatric Surgery, University Hospital,
2Consultant,
3Specialist, Department of Therapy,
4Consultant, Department of Morphology, Faculty of Medicine, University of Medicine,
5Consultant, Private Dental Clinic, Tirana,
6Specialist, Department of Gynecology, Hospital Center, Fier, Albania
Abstract
Background: Adhesion must occur between the demineralized organic mass of the tooth structure and the hydrophilic elements of the adhesive resin. The goal of achieving clinical success with this procedure is clearly expressed in the recommendations for the application of the acidifying and bonding materials, as well as in the rules that appear under the dental material manufacturing brand. Bonding the composite with demineralized dental structures is a procedural technique that relies on the sensitive mechanism of adhesion between the hydrophobic and hydrophilic parts of the resin and the dental organic matrix after acidification. The clinical success of the application is then expressed through the longevity of the filling and the absence of secondary or subsequent complications. Methods: This study is a literature review. A total of 54 articles were selected, of which 12 were excluded from further evaluation as they did not meet one or more of the inclusion criteria. Therefore, 42 articles were included as the base articles for this study. Results: Chlorhexidine (CHX) was applied after rinsing the acid with water and air-drying the dentin only on a wet surface. This method is the only clinically proven one, as it is easy to apply and likely to gain wider acceptance initially. However, research is expected to develop simpler and more efficient methods of applying CHX or other matrix metalloproteinase (MMP) inhibitors. For instance, a recent in vitro study demonstrated that the addition of 2% CHX to a conventional 37% phosphoric acid prevented the degradation of bond strength in an “Etch and Rinse” adhesive system over a six-month period. Conclusions: The clinical application of 2% CHX for one minute on acidified dentin, following an acid rinse and before the application of dentin primer and bond, has been shown to effectively prevent significant in vivo degradation of bond strength by metalloproteinases for a minimum of 14 months.
DOI: 10.32677/yjm.v2i2.3798
Keywords: Chlorhexidine, Degradation, Hybrid layer
Pages: 81-86
View: 2
Download: 3
DOI URL: https://doi.org/10.32677/yjm.v2i2.3798
Publish Date: 28-09-2023
Full Text
INTRODUCTION
The effect of the concentration and time of application of chlorhexidine (CHX) has been evaluated, showing that times less than one minute and concentrations less than 2% can prevent degradation in vitro. However, these results have not yet been verified in vivo. These findings suggest that the success of adding CHX to self-acidification systems to prevent hybrid bond degradation will vary with the resins used in commercial products. In vitro application of concentrations greater than 0.12% CHX to dentin before the primer application is contraindicated, as it may reduce 24-hour bond strength [1–7].
The application of this solution, either in its composition or as part of the primer, highlights the fact that CHX is used in different concentrations for various treatment purposes. This is the element that attracts attention, with the ups and downs in the appearance of the advantages and disadvantages of applying this solution. Furthermore, the theoretical support for the fixation of composite in tooth structures lies in microretention. Regardless of the principles of tooth preparation, tooth structures, such as dentin or enamel, exhibit different hardness and relief roughness. In addition to these characteristics, dentin and demineralized enamel contain numerous “tunnels” and interprismatic spaces and dentinal tubules in quantity, with different numbers per surface unit. The penetration of the adhesive resin into the tubules, opened by acidification, enhances the adhesive surface of the resin to the tooth structure [7–10].
The structure, inorganic content, and organic content of both enamel and dentin undergo specific changes during the initial acidification process and subsequent bonding. The key to the whole repair process with the dental restoration in the tooth lies in the hybrid layer, or the transition layer, from the polymerized adhesive resin to the demineralized layers of the enamel and dentin. This layer plays a vital role in maintaining the integrity of the tissues and in preventing unnecessary structural degradation caused by the activation of enzymes of dissolved collagen.[11–13] A review of the literature about the elements reveals various substances used to inhibit the degradation of the hybrid layer, each differing in their chemical characteristics, such as acid or basic orientation and organic structural composition.
Glutaraldehyde, also known by its trade name Gluma, is one such element applied to prevent degradation of the hybrid layer between dentin and the adhesive material [8,11,14]. Several studies have examined the quantitative and qualitative effects of glutaraldehyde on tooth weight loss, if any, or the release of hydroxyapatite [14–20]. These effects have been assessed at different time points during the clinical lifespan of the completed filling. Therefore, in order to clarify whether degradation of the layer occurs immediately upon acidification or is a time-dependent process, it is important to maintain the stability of collagen fibrils prior to dentin treatment with polyphenols. The application of CHX is one such method [21–25].
The strategy of dentinal bio-modification with proanthocyanidin extracts [21–29] is another source of literature that is oriented around the application of preservation solutions against dentinal degradation. Another strategy is the application of extrafibrillar antimicrobial agents, with kyotosan being one of them, to preserve intrafibrillar minerals within intact collagen fibrils and protease activation. The use of 1% zinc oxide (ZnO) can ensure the integrity of the hybrid layer, reduce cytotoxicity, and minimize the dissolution of the dentinal adhesive [11, 30–37].
Factors that influence the study of this connection can include: [24–34]
• Physical forces of displacement between two solid structures connected by microretention, resulting from occlusal forces. These schooling forces can also express the action as the horizontal component of the occlusal force, applied within the coping norms of the forces on the part of the dental structure.
• Given that the hybrid layer consists of two structures with different dissolution temperatures or undergoing physical shape changes, it is understood that when different temperatures are applied in the connecting contour between the resin and dentine, the reaction of the two-component structures of the hybrid layer is different.
• Substances with different acids or basic contents can penetrate the hybrid layer, which can react and cause the dissolution of the structure between the dentin and the adhesive resin.
• During acidification and bonding, the work protocols, and whether or not moisture is properly maintained, allow excess water in the dentinal fluid. This excess water acts as an initiator for the degradation of the hybrid layer.
• Class I dentinal collagen is broken down by endogenous metalloproteinases or by those of bacterial origin, whose activation occurs and is not hindered by the degradation process of the hybrid layer.
• Different resins have different modes and factors of degradation [21–25].
Moreover, if degradation of the hybrid layer occurs, the stimulus comes from the activation of endogenous metalloproteinases inside the dentinal structure, which destroys the microretention of the filling realized with the composite. The larger the surface to be acidified or bonded, the larger the surface area of the hybrid layer created, and the higher the micro-stress created by the filling [22, 23–29].
MATERIALS AND METHODS
The study focuses on collecting already published data on the effect of CHX on inhibiting hybrid layer degradation. The application of this solution, either simple in composition or as part of a primer, draws attention to the fact that CHX solution is used in different concentrations and for different treatment purposes. This is the element that attracts attention with the ups and downs in the appearance of the advantages and disadvantages of applying this solution [1, 3, 30–38].
The collection of information should be oriented around finding the trend of scientific research in this regard, towards how this data is required through the results according to studies of the review type, or through studies based on experiments performed in vitro or in vivo on permanent teeth during composite restorations [38–43].
The conducted study is a literature review. An electronic search at a time search interval of 15 years was performed on PubMed using the keywords: hybrid layer and CHX and multistep degradation in combination of words [51–86]. After analyzing the collected abstracts and articles, the inclusion and exclusion criteria were applied. The inclusion criteria in the analysis were all the articles that directly evaluated the impact of dentinal biomodification agents on the degradation of the hybrid layer. The exclusion criteria were as follows:
1. Studies that do not directly mention both keywords simultaneously: adaptation of the hybrid
layer and degradation of the hybrid layer.
2. Studies aimed at the strength of the adhesive layer, which had no influence or connection with the hybrid layer.
3. Studies that discussed acidification and bonding of glass-ionomers.
A total of 54 articles and 18 books were initially selected, of which 12 articles were excluded from further evaluation as they did not meet one or some of the following criteria:
1. articles that do not mention periodontal diseases.
2. articles that discuss periodontal diseases, but don’t classify them.
3. articles that do not discuss the treatment of each periodontal disease.
4. articles that discuss not-yet-approved periodontal card systems.
At this stage, 42 articles were selected as part of the basic articles included in the study [51–86].
RESULTS
The goal of achieving clinical success in this procedure is clearly expressed in the recommendations for the application of the acidifying and bonding materials, and the rules provided by the brand of dental material fabrication. The adhesion of the composite to demineralized dental structures is a technical procedure that relies on the sensitive mechanism of adhesion of the hydrophobic and hydrophilic parts of the resin to the organic dentinal matrix after acidification. The clinical success of the application is demonstrated by the longevity of the restorations and the absence of secondary or subsequent complications [37–50]. At the microscopic level, this discussion boils down to analyzing what happens in the hybrid filling layer, which is the initial contact of the adhesive resin with the dentinal tubules or the interprismatic spaces. This contact, depending on the conditions and the level of humidity tolerated by the adhesive resin, following the manufacturer’s recommendations can either remain intact or begin to degrade as an intermediate layer, which will further reflect its effect on the lifespan clinic of the existing filling [43–47].
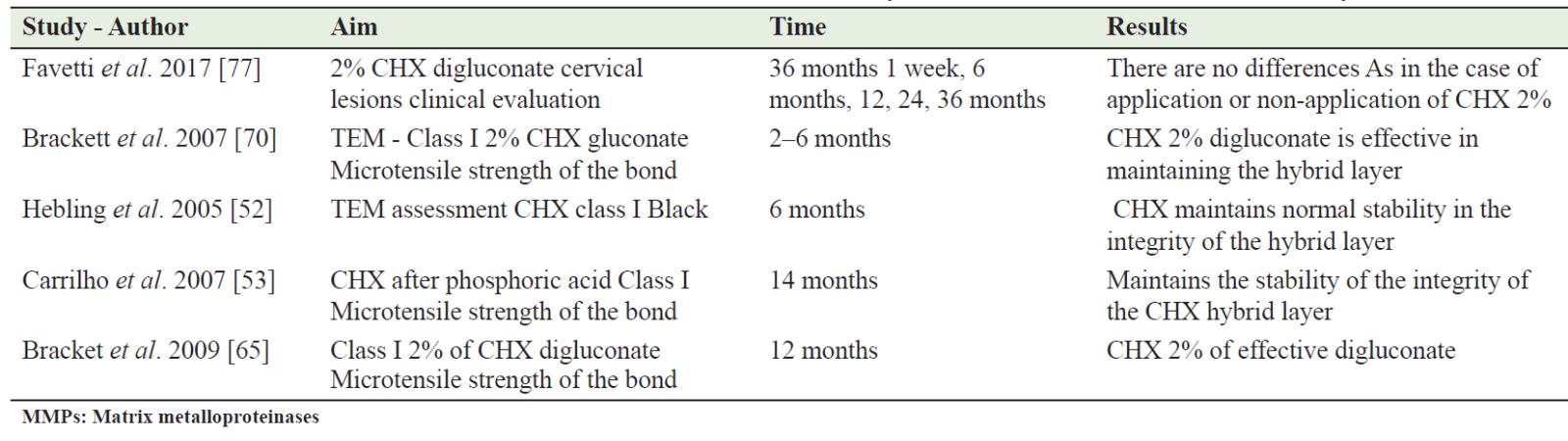
Bonding, as a procedure heavily relies on the method of applying the adhesive layer, whether it is through active or passive application methods. This is primarily oriented by the presence or absence of moisture on the contact surfaces with the demineralized areas. Another element that attracts attention in this second stage of tooth filling is the thickness of the adhesive resin layer in the composite filling. This thickness primarily depends on the thickness of the hybrid layer on the demineralized dentinal structural surfaces, a thickness that is oriented by the penetrating and active ability of the selected acidification type. Table 1 presents all the articles collected in order to understand the trend of scientific research over the years about CHX as an inhibitory solution for MMPs.
Another element has to do with the technical procedure of applying the adhesive resin, which means how much we apply on the demineralized surface of the tooth structure. Here, we intervene to emphasize some recommendations for the application of the air current to orient the mechanical dispersion of the bond on the demineralized dentinal surface of the tooth prepared for filling with composite.
The thickness of the hybrid layer, based on the literature, is highly dependent on the penetration of acidification and the barrier of the adhesive resin against the demineralized dentinal tubules. If we look at this procedure on a microscopic level, it is also related to the process of hydrophobic and hydrophilic interconnections and interactions of the mechanical passage of the adhesive resin against tubules of different dentin sizes. After this transition, the tendency carried out by the force exerted by the air pressure above the non-polymerized layer of the resin adhesive makes another mechanical distribution of this layer against the partially filled dentinal tubules with collagen fibrils, which can even be partially wet. [49–52]
Adhesive materials and their application must be strictly implemented according to the manufacturer’s recommendations. These recommendations, which pertain to the time of application and the technical complexity, are the factors that influence the modification of these materials in specific patients or pediatric patients. These modifications aim to streamline the application process and potentially reduce the overall application time. The reduction of the clinical application time, by reducing a step of the filling with composite, is another advantage of these adhesive materials. Another important element is whether dryness is preserved during the application of the composite filling material. This involves considering whether the material is applied in the presence of moisture or on completely dry demineralized surfaces, i.e., achieving a chalk-like appearance on enamel or a whiter color on demineralized dentinal structures compared to neighboring structures. The method of applying adhesive materials also differs based on the following classification: active and passive application. The difference between these two methods lies in the process of rubbing the material against the dental structures, which is thought to increase the strength of the bond to the hybrid layer. Lastly, the micro-pores caused by demineralization play a crucial role in facilitating the inter-diffusion between the enamel and the dentine hybrid layer. [45–52]
Table 1: All the articles collected to see the trend of scientific research over the years about chlorhexidine as an inhibitory solution of MMPs
DISCUSSION
Based on the sources found in the literature, it can be stated that further studies are needed to assess the degradation of the hybrid layer and obtain long-term clinical data. [34–45] The link between these factors is directly related to the aggressiveness of the acid action, which is expressed in the duration of the acid application and percentage of acidity. Additionally, the depth of the hybrid layer is directly proportional. Consequently, a reduction of the hybrid layer leads to decreased adhesive infiltration and leads to the appearance of fewer partially demineralized areas that are susceptible to hybrid layer degradation [13–17]. Although the demineralizing morphology may be similar for certain types of acidifications, the possibility of creating adaptations of the hybrid layer in the filling and preventing its degradation should be considered.
Depending on the type of acidification, the creation and adaptation of the hybrid layer against the adhesive resin and the dental structure where the filling will be performed exhibit different characteristics [2–5]. The degradation or dissolution of the hybrid layer should be viewed as the process of the collapse of the fiber structure, specifically type I collagen, that remains after acidification. Moreover, it is understood that the degradation of this structure must be seen from the perspective of the activation of collagen-destroying tissue enzymes found in the dentinal tissue matrix. Metalloproteinases and cathepsin cysteine are the enzymes responsible for tissue degradation. When examining the organic content of dentin, it is observed that approximately 90% of the organic part consists of type I collagen, while the remainder comprises non-collagenous proteins. Among these proteins is the group of metalloproteinases, which are thought to be responsible for the degradation of the hybrid layer. These enzymes are sensitive to the presence of certain ions, such as dentinal Zn and Ca. These enzymes are in an inactive form and can be activated locally by cleaving the domer with the catalytic zinc [3–7].
Another aspect of hybrid layer degradation should be considered in terms of the hydrolysis of the adhesive resin, which can be initiated as a result of the application error of the adhesive layer. This is observed more frequently in adhesive systems with multiple application stages compared to systems with a single application [3–7]. Technological advancements aim to prevent the degradation of the hybrid layer, as it gradually leads to the development of complications in composite fillings [7]. The degradation of the hybrid layer occurs as a result of the hydrolysis of the adhesive resin and the dissolution of collagen, both components of the hybrid layer. The hydrolytic stability of the hybrid layer differs from the application technique of the adhesive layer, thereby expressing higher results in systems with different stages of application than in systems with united stages of application. The breakdown of collagen and the release of collagen type 1 occurs due to the activation of metalloproteinases and cysteine cathepsin, which are responsible for the hydrolytic degradation of the collagenous part of the hybrid layer. After the demineralization of the dentin during acidification, the moisture of the rinsing process takes place in the preservation of the collagenous structure. Additionally, the resin cannot remove water residues but leaves behind layers of demineralization residues, which fall prey to the hydrolytic degradation of the hybrid layer, after the activation of metalloproteinases.
Santos et al. considered that 2% CHX, either in aqueous solution or gel form, as a non-oxidizing agent, did not interfere with the interaction of an adhesive system in pulp chamber dentin. However, an exception to this trend was observed when CHX gel was combined with Ethylenediaminetetraacetic acid (EDTA). Although the base of the gel used with CHX is a water-soluble carbon polymer, which can be easily removed from the root canal, an occasional presence of residual gel in the dentin may react with EDTA. This reaction can lead to the formation of products that affect resin infiltration and/or polymerization, resulting in slightly lower bond strength values compared to the control group. Therefore, it is important to make every effort to remove traces of chemical substances from the canal through intermediate rinses with inert solutions [49–52]. CHX has demonstrated the ability to maintain hybrid layer stability and bond strength in both vitro and in vivo studies, possibly due to its effectiveness as an MMP inhibitor. This inhibitory effect results in reduced degradation of the hybrid layer and collagen fibrils. It is a remarkable property because one of the reasons for the long-term loss of integrity in composite-dentin bonds is the degradation of collagen fibrils exposed in incomplete infiltrated hybrid layers. This degradation is attributed to an endogenous proteolytic mechanism involving the activity of MPMs present in dentin [20–22].
CONCLUSION
The application of CHX and its significance in preventing the degradation of the hybrid layer have been confirmed in the published sources found in the literature. The method of its application is easy as an application procedure. In the future, adhesive systems that allow the gradual release of CHX over an extended period will be developed. According to the literature, the effects of CHX can be sustained even up to 10 years after application. However, it is worth noting that there are relatively few literature sources and studies in this field, and they are primarily focused on in vitro research using extracted teeth rather than in vivo studies on natural teeth.
AUTHORS’ CONTRIBUTION
All authors have made a significant contribution to this work, be it in the conception, implementation, and literature review or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be responsible for all aspects of the work.
References
- Venigalla BS, Jyothi P, Kamishetty S, et al. Resin bond strength to water versus ethanol-saturated human dentin pretreated with three different cross-linking agents. J Conserv Dent. 2016;19(6):555-559.
- Zecin-Deren A, Sokolowski J, Szczesio-Wlodarczyk A, et al. Multi-layer application of self-etch and universal adhesives and the effect on dentin bond strength. Molecules. 2019;24(2):345.
- Robo I, Heta S, Gjumsi E, et al. Oral Microflora, in Cases with Gingival Hypertrophy Caused by Fixed Orthodontic Appliances. SN Comprehensive Clinical Medicine. 2021;3.1-9.
- Frassetto A, Breschi L, Turco G, et al. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability--A literature review. Dent Mater. 2016;32(2):e41-53.
- Li Y, Hu X, Xia Y, et al. Novel magnetic nanoparticle-containing adhesive with greater dentin bond strength and antibacterial and remineralizing capabilities. Dent Mater. 2018;34(9):1310-1322.
- Cecchin D, Farina AP, Vidal C, et al. A novel enamel and dentin etching protocol using α-hydroxy glycolic acid: surface property, etching pattern, and bond strength studies. Oper Dent. 2018;43(1):101-110.
- Vasconcelos E Cruz J, Polido M, Brito J, et al. Dentin bonding and SEM analysis of a new experimental universal adhesive system containing a dendrimer. Polymers (Basel). 2020;12(2):461.
- Botelho MPJ, Isolan CP, Schwantz JK, et al. Rubbing time and bonding performance of one-step adhesives to primary enamel and dentin. J Appl Oral Sci. 2017;25(5):523-532.
- Prati C, Nucci C, Toledano M, et al. Microleakage and marginal hybrid layer formation of compomer restorations. Oper Dent. 2004;29(1):35-41.
- Perdigão J. Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent Mater. 2010;26(2):e24-37.
- Gibby SG, Wong Y, Kulild JC, et al. Novel methodology to evaluate the effect of residual moisture on epoxy resin sealer/dentine interface: a pilot study. Int Endod J. 2011;44(3):236-44.
- Prati C, Chersoni S, Acquaviva GL, et al. Permeability of marginal hybrid layers in composite restorations. Clin Oral Investig. 2005;9(1):1-7.
- Onay EO, Orucoglu H, Kiremitci A, et al. Effect of Er,Cr:YSGG laser irradiation on the apical sealing ability of AH Plus/gutta-percha and Hybrid Root Seal/Resilon Combinations. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2010;110(5):657-64.
- Hossain M, Yamada Y, Nakamura Y, et al. A study on surface roughness and microleakage test in cavities prepared by Er:YAG laser irradiation and etched bur cavities. Lasers Med Sci. 2003;18(1):25-31.
- Rontani RM, Ducatti CH, Garcia-Godoy F, et al. Effect of etching agent on dentinal adhesive interface in primary teeth. J Clin Pediatr Dent. 2000;24(3):205-9.
- Sauro S, Mannocci F, Toledano M, et al. EDTA or H3PO4/NaOCl dentine treatments may increase hybrid layers' resistance to degradation: a microtensile bond strength and confocal-micropermeability study. J Dent. 2009;37(4):279-88.
- Wang JH, Yang K, Zhang BZ, et al. Effects of Er:YAG laser pre-treatment on dentin structure and bonding strength of primary teeth: an in vitro study. BMC Oral Health. 2020;20(1):316.
- Hirabayashi S, Yoshida E, Hayakawa T. SEM analysis of microstructure of adhesive interface between resin cement and dentin treated with self-etching primer. Dent Mater J. 2011;30(4):528-36.
- Baseggio W, Consolmagno EC, de Carvalho FL, et al. Effect of deproteinization and tubular occlusion on microtensile bond strength and marginal microleakage of resin composite restorations. J Appl Oral Sci. 2009;17(5):462-6.
- Zhang Y, Wang Y. Distinct photopolymerization efficacy on dentin of self-etch adhesives. J Dent Res. 2012;91(8):795-9.
- Saikaew P, Chowdhury AF, Fukuyama M, et al. The effect of dentine surface preparation and reduced application time of adhesive on bonding strength. J Dent. 2016;47:63-70.
- Lin J, Zheng WY, Liu PR, et al. Influence of casein phosphopeptide-amorphous calcium phosphate application, smear layer removal, and storage time on resin-dentin bonding. J Zhejiang Univ Sci B. 2014;15(7):649-60.
- Sundfeld RH, Valentino TA, de Alexandre RS, et al. Hybrid layer thickness and resin tag length of a self-etching adhesive bonded to sound dentin. J Dent.2005;33(8):675-81.
- Mazzoni A, Carrilho M, Papa V, et al. MMP-2 assay within the hybrid layer created by a two-step etch-and-rinse adhesive: biochemical and immunohistochemical analysis. J Dent. 2011;39(7):470-7.
- Leme AA, Vidal CM, Hassan LS, et al. Potential role of surface wettability on the long-term stability of dentin bonds after surface biomodification. J Biomech. 2015;48(10):2067-71.
- Boruziniat A, Gharaei S. Bond strength between composite resin and resin modified glass ionomer using different adhesive systems and curing techniques. J Conserv Dent. 2014;17(2):150-4.
- Cai J, Palamara JEA, Burrow MF. Effects of collagen crosslinkers on dentine: A literature review. Calcif Tissue Int. 2018;102(3):265-279.
- Arrais CA, Miyake K, Rueggeberg FA, et al. Micromorphology of resin/dentin interfaces using 4th and 5th generation dual-curing adhesive/cement systems: a confocal laser scanning microscope analysis. J Adhes Dent. 2009;11(1):15-26.
- Wagner A, Wendler M, Petschelt A, et a. Bonding performance of universal adhesives in different etching modes. J Dent. 2014;42(7):800-7.
- Dos Santos PH, Karol S, Bedran-Russo AK. Long-term nano-mechanical properties of biomodified dentin-resin interface components. J Biomech. 2011;44(9):1691-4.
- Leme-Kraus AA, Phansalkar RS, Dos Reis MC, et al. Dimeric proanthocyanidins on the stability of dentin and adhesive biointerfaces. J Dent Res. 2020;99(2):175-181.
- Bedran-Russo AK, Pauli GF, Chen SN, et al. Dentin biomodification: strategies, renewable resources, and clinical applications. Dent Mater. 2014;30(1):62-76.
- Bedran-Russo AK, Zamperini CA. New preventive approaches part II: Role of dentin biomodifiers in caries progression. Monogr Oral Sci. 2017;26:97-105.
- Moreira MA, Souza NO, Sousa RS, et al. Efficacy of new natural biomodification agents from Anacardiaceae extracts on dentin collagen cross-linking. Dent Mater. 2017;33(10):1103-1109.
- Anshida VP, Kumari RA, Murthy CS, et al. Extracellular matrix degradation by host matrix metalloproteinases in restorative dentistry and endodontics: An overview. J Oral MaxillofacPathol. 2020;24(2):352-360.
- Castellan CS, Bedran-Russo AK. Biomimetic strategy to stabilize the mechanical properties of caries-affected dentin matrix: A 12-month in vitro study. Am J Dent. 2020;33(2):64-68.
- Liu RR, Fang M, Zhang L, et al. Anti-proteolytic capacity and bonding durability of proanthocyanidin-biomodified demineralized dentin matrix. Int J Oral Sci. 2014;6(3):168-74.
- Sun Q, Gu L, Wu S, et al. Biomodifying effect of epigallocatechin-3-gallate on dentine substrate splicing surface. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016;51(3):148-53.
- Hiraishi N, Sono R, Sofiqul I, et al. In vitro evaluation of plant-derived agents to preserve dentin collagen. Dent Mater. 2013;29(10):1048-54.
- Vidal CM, Aguiar TR, Phansalkar R, et al. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014;10(7):3288-94.
- Prakki A, Xiong Y, Bortolatto J, et al. Functionalized epigallocatechin gallate copolymer inhibit dentin matrices degradation: Mechanical, solubilized telopeptide and proteomic assays. Dent Mater. 2018;34(11):1625-1633.
- Nam JW, Phansalkar RS, Lankin DC, et al. Absolute configuration of native oligomeric proanthocyanidins with dentin biomodification potency. J Org Chem. 2017;82(3):1316-1329.
- Venigalla BS, Jyothi P, Kamishetty S, et al. Resin bond strength to water versus ethanol-saturated human dentin pretreated with three different cross-linking agents. J Conserv Dent. 2016;19(6):555-559.
- Moraes IQS, Nascimento TG, Silva AT, et al. Inhibition of matrix metalloproteinases: a troubleshooting for dentin adhesion. Restorative Dentistry & Endodontics 2020;45(3).
- Mazzoni A, Tjäderhane L, Checchi V, et al. Role of dentin MMPs in caries progression and bond stability. J Dent Res. 2015;94(2):241-51.
- Göstemeyer G, Schwendicke F. Inhibition of hybrid layer degradation by cavity pretreatment: Meta- and trial sequential analysis. J Dent. 2016;49:14-21.
- Breschi L, Maravic T, Comba A, et al. Chlorhexidine preserves the hybrid layer in vitro after 10-years aging. Dent Mater. 2020;36(5):672-680.
- Tjäderhane L, Nascimento FD, Breschi L, et al. Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent Mater. 2013;29(10):999-1011.
- Vallabhdas AK, Kumar CNV, Kabbinale P, et al. Evaluation of hybrid layer and bonding interface after water storage with and without the usage of 2% chlorhexidine: A Scanning Electron Microscope Study. J Contemp Dent Pract. 2018;19(1):52-59.
- Moon PC, Weaver J, Brooks CN. Review of matrix metalloproteinases' effect on the hybrid dentin bond layer stability and chlorhexidine clinical use to prevent bond failure. Open Dent J. 2010;4:147-52.
- Strobel S, Hellwig E. The effects of matrix-metallo- proteinases and chlorhexidine on the adhesive bond. Swiss Dent J. 2015;125(2):134-45.
- Hebling J, Pashley DH, Tjäderhane L, et al. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84(8):741-6.
- Carrilho MR, Geraldeli S, Tay F, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86(6):529-33.
- Collares FM, Rodrigues SB, Leitune VC, et al. Chlorhexidine application in adhesive procedures: a meta-regression analysis. J Adhes Dent. 2013;15(1):11-8.
- Lingling J, Qianbing W. Progress on matrix metalloproteinase inhibitors. iApr 2017;35(2):208-214.
- Ou Q, Hu Y, Yao S, et al. Effect of matrix metalloproteinase 8 inhibitor on resin-dentin bonds. Dent Mater. 2018;34(5):756-763.
- Gou YP, Li JY, Meghil MM, et al. Quaternary ammonium silane-based antibacterial and anti-proteolytic cavity cleanser. Dent Mater. 2018;34(12):1814-1827.
- Kiuru O, Sinervo J, Vähänikkilä H, et al. MMP Inhibitors and Dentin Bonding: Systematic Review and Meta-Analysis. Int J Dent. 2021;27;2021:9949699.
- Zhang SC, Kern M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int J Oral Sci. 2009;1(4):163-76.
- Baldion PA, Betancourt DE. Dataset on the effect of flavonoids on the stabilization of the resin-dentin interface. Data Brief. 2021;35:106984.
- Li H, Li T, Li X, et al. Morphological effects of MMPs inhibitors on the dentin bonding. Int J Clin Exp Med. 2015;8(7):10793-803.
- Li F, Majd H, Weir MD, et al. Inhibition of matrix metalloproteinase activity in human dentin via novel antibacterial monomer. Dent Mater. 2015;31(3):284-92.
- Fialho MPN, Hass V, Nogueira RP, et al. Effect of epigallocatechin-3- gallate solutions on bond durability at the adhesive interface in caries-affected dentin. J Mech Behav Biomed Mater. 2019;91:398-405.
- Breschi L, Martin P, Mazzoni A, et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater. 2010;26(6):571-8.
- Brackett MG, Tay FR, Brackett WW, et al. In vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Oper Dent. 2009;34(4):379-83.
- de Menezes LR, da Silva EO, Maurat da Rocha LV, et al. The use of clays for chlorhexidine controlled release as a new perspective for longer durability of dentin adhesion. J Mater Sci Mater Med. 2019;30(12):132.
- Sadek FT, Braga RR, Muench A, et al. Ethanol wet-bonding challenges current anti-degradation strategy. J Dent Res. 2010;89(12):1499-504.
- Pinheiro SL, Pereira DR, De Milito F, et al. Influence of metalloproteinases on dentin hybridization of one-bottle or self-etch dental bonding systems. J Contemp Dent Pract. 2014;15(6):705-11.
- Silva MDS, Neto NL, da Costa SA, et al. Biophysical and biological characterization of intraoral multilayer membranes as potential carriers: A new drug delivery system for dentistry. Mater Sci Eng C Mater Biol Appl. 2017;71:498-503.
- Brackett WW, Tay FR, Brackett MG, et al. The effect of chlorhexidine on dentin hybrid layers in vivo. Oper Dent. 2007;32(2):107-11.
- Brackett MG, Li N, Brackett WW, et al. The critical barrier to progress in dentine bonding with the etch-and-rinse technique. J Dent. 2011;39(3):238-48.
- Sabatini C, Ortiz PA, Pashley DH. Preservation of resin-dentin interfaces treated with benzalkonium chloride adhesive blends. Eur J Oral Sci. 2015;123(2):108-15.
- Epasinghe DJ, Yiu CK, Burrow MF, et al. The inhibitory effect of proanthocyanidin on soluble and collagen-bound proteases. J Dent. 2013;41(9):832-9.
- Pashley DH, Tay FR, Imazato S. How to increase the durability of resin-dentin bonds. Compend Contin Educ Dent. Sep;32(7):60-4, 66, 2011.
- Gou Y, Jin W, He Y, et al. Effect of cavity cleanser with long-term antibacterial and anti-proteolytic activities on resin-dentin bond stability. Front Cell Infect Microbiol. 2021;11:784153.
- De Munck J, Mine A, Van den Steen PE, et al. Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. Eur J Oral Sci. 2010;118(5):494-501.
- Favetti M, Schroeder T, Montagner AF, et al. Effectiveness of pre-treatment with chlorhexidine in restoration retention: A 36-month follow-up randomized clinical trial. J Dent. 2017;60:44-49.
- Porto ICCM, Nascimento TG, Oliveira JMS, et al. Use of polyphenols as a strategy to prevent bond degradation in the dentin-resin interface. Eur J Oral Sci. 2018;126(2):146-158.
- Thompson JM, Agee K, Sidow SJ, et al. Inhibition of endogenous dentin matrix metalloproteinases by ethylenediaminetetraacetic acid. J Endod. 2012;38(1):62-5.
- Scheffel DL, Hebling J, Scheffel RH, et al. Inactivation of matrix-bound matrix metalloproteinases by cross-linking agents in acid-etched dentin. Oper Dent. 2014;39(2):152-8.
- Tay FR, Pashley DH, Loushine RJ, et al. Self-etching adhesives increase collagenolytic activity in radicular dentin. J Endod. 2006; 32(9):862-8.
- Leitune VC, Portella FF, Bohn PV, et al. Influence of chlorhexidine application on longitudinal adhesive bond strength in deciduous teeth. Braz Oral Res. 2011;25(5):388-92.
- Stanislawczuk R, Reis A, Loguercio AD. A 2-year in vitro evaluation of a chlorhexidine-containing acid on the durability of resin-dentin interfaces. J Dent. 2011;39(1):40-7.
- Liu N, Li F, Chen YJ, et al. Effect of quaternary ammonium methacrylates incorporation into a dental adhesive on the resistance of enzymatic degradation of resin-dentine interfaces. Zhonghua Kou Qiang Yi Xue Za Zhi. 2013;48(7):414-8.
- Tezvergil-Mutluay A, Agee KA, Uchiyama T, et al. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90(4):535-40.
- Tezvergil-Mutluay A, Agee KA, Hoshika T, et al. The inhibitory effect of polyvinylphosphonic acid on functional matrix metalloproteinase activities in human demineralized dentin. Acta Biomater. 2010;6(10):4136-42.
