Full HTML
Hypothermia therapy in neonatal hypoxic-ischemic encephalopathy: Current perspectives, combination therapy and future directions
Moaaz Abo Zeed 1*, Maher Mohamad Najm 2,3, Arwa Ajaj 4, Mohamad Ahmad Ajaj 5
Author Affiliation
1Neonatal Intensive Care Unit, Women's Wellness and Research Center, Hamad Medical Corporation, Doha, Qatar
2,3 Pediatric Emergency Center, Hamad Medical Corporation, Doha, Qatar,
4Sidra Medicine, Ar-Rayyan, Qatar
5Shmaisani Hospital, University of Jorden, Jordan
Abstract
Hypoxic-ischemic encephalopathy (HIE) in neonates, resulting from oxygen deprivation during birth, is a significant cause of death and long-term disabilities. Therapeutic hypothermia has emerged as a pivotal intervention for improving neurological outcomes in infants with HIE. This review aims to summarize current practices, outcomes, and challenges of hypothermia therapy in neonatal HIE, and adjuvant therapies, along with future directions in this evolving field.
DOI: 10.18231/j.yjom.2024.019
Keywords: Therapeutic hypothermia, Hypoxicischemic encephalopathy, Cooling, Neonatal neuroprotection
Pages: 182-189
View: 42
Download: 59
DOI URL: https://doi.org/10.18231/j.yjom.2024.019
Publish Date: 15-12-2024
Full Text
Hypoxic-ischemic encephalopathy (HIE) is the suppression of brain activity with brain injury due to inadequate oxygen (hypoxia) or perfusion (ischemia) to the brain. HIE is a significant cause of neonatal mortality and morbidity worldwide. 10% to 60% of affected newborns die, and neurodevelopmental sequelae occur in at least 25% of survivors. 1,2 Adverse outcomes include developmental delay or intellectual impairment, cerebral palsy, epilepsy, sensorineural deafness, and blindness. The incidence of (HIE) is 1-2 per 1,000 live births in the Western world and is far more common in resource-limited countries, at 5– 40 in every 1,000 births. 3,4
The complicated pathophysiology of HIE consists of a primary (acute) phase, a secondary phase (latent), and a tertiary phase. It is further classified based on the severity into mild, moderate, or severe encephalopathy according to the degree of brain injury and neurological manifestations, which are examined and documented by medical staff using the Sarnat staging or Thompson score. 5–8 Several interventions have been explored to manage HIE. Therapeutic hypothermia (TH) is now the standard of care for newborns with moderate to severe HIE as proven in high-quality randomized controlled Trials (RCT) and metaanalyses. 2,9–16 A statistically and clinically significant reduction of 25 % in the combined outcome of mortality or major neurodevelopmental disability at age 18 months was reported after cooling in Cochrane meta-analysis. 2 Although cooling is beneficial, its efficacy is still limited, so the need for other neuroprotective strategies and therapies is essential. Despite we do not yet know which therapy works best in combination or whether these therapies are safe, the addition of other neuroprotective strategies may potentially improve the outcome. Many pharmacological treatment options are being studied in conjunction with TH (e.g. erythropoietin, allopurinol, melatonin, cannabidiol, exendin-4/exenatide), but still are not standard of care. 17–19
Mechanism of therapeutic hypothermia
The primary mechanism resulting in brain damage after intrapartum hypoxia-ischemia is diminished cerebral blood flow in a hypoxic environment. [20] Two stages of energy failure are caused by hypoxia-ischemia at the cellular level. The primary phase occurs after brain hypoperfusion and deprivation of oxygen, with a decrease in adenosine triphosphate (ATP), failure of the sodium (Na+)/potassium (K+) pump, depolarization of cells, lactic acidosis, release of excitatory amino acids, calcium entry into the cell and, when severe, cell death. 21 Prior to irreversible mitochondrial function failure, there A. Umbilical Cord pH ≤7.0 or base deficit ≥ −16, OR B. pH 7.01 to 7.15 or base deficit −10 to −15.9 on cord gas or blood gas within 1 hr AND 1. History of an acute perinatal event (such as but not limited to cord prolapse, placental abruption, or uterine rupture) AND, 2. Apgar score ≤5 at 10 minutes or at least 10 minutes of positive-pressure ventilation C. Evidence of moderate-to-severe encephalopathy, demonstrated by the presence of seizures OR at least one sign in three or more of the six categories. (Table 1) Table 1: Criteria for defining moderate and severe encephalopathy is a latent period of one to six hours after neonatal resuscitation and reperfusion of the brain during which the Category Moderate encephalopathy Severe encephalopathy impairment of cerebral oxidative metabolism can at least partially recover. The window of opportunity for neuroprotective treatments including TH is during this latent Level of consciousness Spontaneous activity Lethargy Stupor/coma Decreased activity No activity window which is most likely in 1st six hours after birth. [21,22]
Selection criteria of newborns with HIE who should receive TH
According to data from major RCTs, TH should be offered to all babies with moderate to severe HIE who fulfill certain criteria. 11–16 American Academy of Pediatrics (AAP) set the following criteria. 23 Posture Distal flexion, full extension Tone Hypotonia (focal, general) Primitive reflexes Decerebrate (arms extended and internally rotated, legs extended with feet in forced plantar flexion) Flaccid
1. The newborn is more than 35 weeks gestational age (GA) and less than 6 hours of age.
2. “Proof of asphyxia,” as defined by the presence of at least one to two of the following:
- (a) Apgar score less than 6 at 10 minutes or continued need for resuscitation with positive pressure ventilation or chest compressions at 10 minutes.
- (b) Any acute perinatal sentinel event that may result in HIE (e.g., significant fetal heart rate abnormality, cord prolapse, abruptio placentae).
- (c) Umbilical Cord pH less than 7.0 or base excess of −16 mmol/L or less. (d) If cord pH is not available, arterial pH less than 7.0 or base excess less than −16 mmol/L within 60 minutes of birth
3. Evidence of moderate/severe neonatal encephalopathy on clinical examination (modified Sarnat score). 7
Pediatric Canadian Society (PCS) guidelines state that infants who may benefit from TH are term and late preterm infants ≥36 weeks GA with HIE who are ≤6 hours old and who meet either treatment criteria A or treatment criteria B, and meet criteria C. [20]
A. Umbilical Cord pH ≤7.0 or base deficit ≥ −16, OR
B. pH 7.01 to 7.15 or base deficit −10 to −15.9 on cord gas or blood gas within 1 hr AND
- 1. History of an acute perinatal event (such as but not limited to cord prolapse, placental abruption, or uterine rupture) AND,
- 2. Apgar score ≤5 at 10 minutes or at least 10 minutes of positive-pressure ventilation
C. Evidence of moderate-to-severe encephalopathy, demonstrated by the presence of seizures OR at least one sign in three or more of the six categories. (Table 1)
Table 1: Criteria for defining moderate and severe encephalopathy
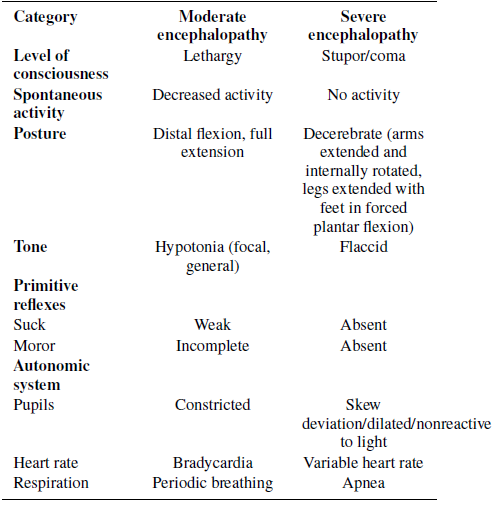
Using an amplitude-integrated electroencephalogram (aEEG) is a very helpful tool to decide on initiating TH. 2.3. Therapeutic hypothermia exclusion criteria Exclusion criteria in RCTs comprised moribund infants, major congenital or chromosomal abnormalities for whom no further aggressive treatment is planned, clinically significant coagulopathy or bleeding despite treatment, evidence of severe head trauma or intracranial hemorrhage and overwhelming septicemia. 2,11–16
Mild TH with a target core temperature of 33.5◦C ± 0.5◦C (rectal or esophageal) can be provided through whole- body or selective head cooling. When both approaches were compared to a placebo, main clinical trials revealed comparable effects on death and disability outcomes. 9 Head cooling can be accomplished with cooling caps fixed around the newborn head and targeting fontanelle temperature below 30◦C and rectal temperature of 33.5◦C ± 0.5◦C which, can be achieved by using a servo-controlled radiant heater. This method is more complex and expensive than body cooling devices, in addition, manual adjustments of radiant warmers are required. Other limitations include inducing scalp edema or skin injury, difficulty maintaining rectal temperature, and limited access to EEG leads.
On the other hand, whole-body cooling is more widely used and recommended by neonatal professionals. Cooling temperature can be reached by passive cooling, ice packs or cool gels, and/or commercial cooling blankets can all help cool the entire body. Target temperatures are more reliably regulated by cooling blankets that are servo-controlled. [24] The target core temperature (esophageal or rectal) should be kept at 33–34 ◦C for 72 hours for patients undergoing TH according to several groups’ recommendations. 2,11–16
Rewarming must be performed gradually and slowly, with a maximum increase in core temperature of 0.5 ◦C per hour until normothermia is attained at 37 ◦C. Stepwise temperature increases are avoided with the automatic rewarming modes found in more recent servo-controlled systems.
Access and resource limitations
TH should be provided in Level 3 and 4 neonatal units as those facilities recruit medical staff who are competent and knowledgeable about the complexities of treatment, in addition to all needed equipment. Any newborn with HIE with a potential need for cooling who is born in low-level care centers must be transferred to an advanced unit for further management.
Over 60% of registered neonates were outborn babies, according to data from the Vermont Oxford Encephalopathy Registry. 25 The start of passive cooling in a community hospital while awaiting transport to a tertiary care unit should be considered after consulting with a neonatologist. The infant’s blanket, hat, and overhead warmer can be removed, and ice packs or cool gels can be applied. To make sure the baby’s temperature doesn’t drop below 33◦C, the temperature should be checked every 15 minutes rectally, if that’s not feasible, monitor axillary temperature. [26]
Monitoring and side effects of cooling
Continuous monitoring during TH is crucial to manage potential complications. RCTs reported many adverse effects including cardiac arrhythmias and most commonly sinus bradycardia (a heart rate of less than100 beats/minute), hypotension with possible need for inotropes, persistent pulmonary hypertension, thrombocytopenia, anemia, leukopenia, coagulopathy, urinary retention. Hypoglycemia, hypokalemia, and subcutaneous fat necrosis, with or without hypercalcemia which are rare complications. 26,27
Indications to discontinue therapeutic Hypothermia prematurely and re-warm 23
- 1. Persistent Pulmonary Hypertension of the Newborn with refractory hypoxemia despite maximal medical therapy with fraction of inspired oxygen (FiO2) > 0.8 (Due to the shift in the oxyhemoglobin dissociation curve to the left with hypothermia).
- 2. Contractable hypotension despite using inotropic support.
- 3. Clinically significant coagulopathy despite treatment.
- 4. Severe intracranial hemorrhage as cooling has not proven to be beneficial and may worsen coagulopathy in infants who suffered from this complication.
- 5. Subcutaneous fat necrosis, with or without hypercalcemia.
Expanding TH Eligibility criteria
1. Cooling babies with mild HIE Studies of TH accommodated neonates with moderate and severe HIE and those with mild HIE 2,11−16. According to retrospective studies, some infants who did not undergo cooling experienced negative short-term outcomes, such as seizures, feeding difficulties, and abnormal magnetic resonance imaging (MRI). 28–30 These findings led some centers to administer cooling to neonates exhibiting mild HIE despite a lack of clinical trials supporting such intervention. There is little prospective data on mild HIE identified at less than six hours of age, and the basic question of how to best manage mild HIE is still open. Many RCTs investigating mild HIE management are underway with the hope to obtain supporting treatment evidence.
2. Initiation of cooling beyond 6 hours after birth All studies emphasized that hypothermia should be started as early as possible in the first 6 hours of life to achieve potential benefits and best outcomes. 2,11–16 However, cooling of some infants cannot be started during this window due to many reasons such as delayed diagnosis or being born in places that cannot support cooling. One large RCT was published in 2017 included 168 term neonates with HIE who underwent TH between > 6 - 24 hours of life and randomized to TH or normothermia and no significant difference between the two groups regarding neurodevelopmental outcome which was assessed in survivors at 18–22 months. [31] For the best chance of a successful outcome, this study reaffirms the necessity of robust clinical protocols that guarantee TH is initiated within the first six hours of life for eligible infants with HIE.
3. Cooling of preterm infants A higher risk of death in preterm infants is linked to TH. Data is very limited about using TH in neonates less than 35 weeks and a small number of those infants were included in some clinical studies. So, the current evidence does not support providing TH for such populations. [32,33]
Applying cooling therapy in low- and middle-income countries (LMICs)
Neonatal encephalopathy is thought to be the cause of about one million newborn deaths annually in low- and middleincome nations. 34,35 A large, rigorous, randomized study (HELIX trial) was published in 2021. The results do not support the use of TH among infants with HIE in LMICs and intervention surprisingly increased the rate of mortality in treated infants. However, this was a single study that was not representative of low- and middle- income countries worldwide and was heavily weighted for India’s representation. 36 HELIX trail has been combined with nine other smaller RCTs of TH conducted in LMICs in a metaanalysis (n = 1131 infants), and the authors concluded that in neonates with HIE in LMICs, TH most likely has little to no impact on clinical outcomes. 37 To clarify the usefulness of TH in settings with limited resources, more extensive research focusing on appropriate patient selection is required.
Precision medicine in HIE
Using artificial intelligence (AI) in medicine is rapidly evolving. AI and Machine Learning (ML) could be useful if utilized in neonatal medicine. ML builds models or algorithms by analyzing data to find trends and patterns by learning from past experiences. Outcome predictions can be made based on these algorithms, which subsequently help in decision-making and parent counseling. In neonates with HIE, creating new tools for a precision medicine approach based on big databases and ML applied to neuromonitoring, neuroimaging data, genetic analysis, and assays measuring multiple biomarkers (omics) could be one way to identify infants who will develop neurodevelopmental delay and other neurological outcomes accurately shortly after birth. [38–40]
Combined therapy
Despite the high level of evidence in the literature on the effectiveness of TH in HIE, the percentage of infants developing neurodevelopmental complications (including cognitive and motor impairment) post-HIE in those who received TH is still high. 41,42 Moreover, as discussed above, the benefit of TH is limited to a certain population, which makes the management of HIE more challenging in preterm neonates (born at gestational age of <35 weeks), neonates born in LMICs, or those who failed to be started on cooling therapy before 6 hours after birth, etc. This highlights the importance of investigating and understanding the effectiveness and safety of other potential neuroprotective therapies for HIE, whether as mono- or adjunct therapy. Therapies that were proposed in the literature and those that will be discussed in this review include erythropoietin, magnesium sulfate, melatonin, xenon, and stem cells.
1. Erythropoietin
Erythropoietin (EPO) is an endogenous hormone produced in the kidney and is primarily known for its role in erythropoiesis and there are FDA-approved recombinant forms, such as Darbepoetin that are usually used for the treatment of anemia. 43,44 However, it is also known as pleiotropic cytokines, and its role is not limited only to hematopoietic cells as it was also found to have a neuroprotective effect based on pre-clinical studies (in vitro and in vivo studies). 45–47 EPO’s neuroprotective role was attributed to its anti-apoptotic, anti-neurotoxicity, and antiinflammatory effects; in addition, it also prevents white matter injury and cerebral edema and promotes neural regeneration. [47]
EPO was proposed as a potential treatment for HIE as either monotherapy or in combination with TH. Published RCTs have studied the efficacy and safety of EPO at different doses when used in combination with TH. These studies have reported that the combination of EPO has significantly reduced the short-term rate of mortality, 48,49 neurodevelopmental impairment and disability. 49–51 It also reduced the level of brain injury biomarkers in the CSF, 52 ECG changes, 51 and brain MRI changes suggesting severe brain injuries. 51 It was also found to be an effective therapy in terms of reducing neurodevelopmental disability when used as monotherapy to treat HIE in LMICs, as evidenced by two systemic reviews 53 and one RCT. 50 Overall, when used as either monotherapy or as a combination therapy, no significant adverse effect was noted in the aforementioned RCTs and systemic reviews, and it was reported that EPO was generally safe. However, the level of evidence provided by these studies is low, given the small sample size and lack of a control group in certain studies. In addition, the promising outcomes that were reported were based on a short-term period as neonates were followed for a maximum of 12 to 18 months.
Interestingly, more recent and higher evidence papers were done on a larger scale and included multicenter and double-blinded RCT to investigate the efficacy and safety of EPO in combination with TH in HIE. High-dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) trial, a large multicenter, phase 3 double-blinded RCT included five hundred neonates with moderate or severe HIE who are being treated with TH in combination with high dose EPO (1,000 U/kg) or placebo (normal saline). 54 The HEAL trial concluded that the combination of EPO with TH in HIE, in contrast to the preclinical studies and small-sized phase 2 trials, did not decrease the rate of mortality, neurodevelopmental impairment, or risk of brain injury. 54 A sub-analysis of the same trial also reported that such a combination did not reduce the risk of seizure in HIE neonates. 55 In addition, upon further analysis, the trial also demonstrated that the use of EPO as an adjuvant therapy in neonates with HIE was not associated with lower levels of neuroinflammation or brain injury biomarkers. 56 These biomarkers are an important prognostic factor as low levels are strongly associated with lower mortality and neurodevelopmental impairment at 2 years, in contrast to clinical assessment only. 56 In contrast to the previous systemic analysis and RCT suggesting EPO monotherapy as a possible effective and safe treatment for the management of HIE, a more recent systemic review and meta-analysis does not recommend the use of EPO at any dose as either mono- or adjunct therapy given the lack of its efficacy (i.e. did not decrease mortality or risk of neurodevelopmental impairment). 57 The use of EPO, as evidenced by the HEAL trial and a systemic review with meta-analysis, was not completely safe as it was associated with adverse events such as thrombosis. [54,56]
In conclusion, EPO was proposed as a potential therapy in managing neonates with HIE based on preclinical studies and early-phase clinical trials. However, based on higher evidence studies and trials, there is no strong evidence of the efficacy and safety to support the use of EPO in HIE. Nevertheless, overall, there is still limited evidence in the literature, and more studies are recommended to be done on a larger scale in terms of sample size, duration, and population.
2. Magnesium sulfate Magnesium sulphate (MgSO4) is one of the wildly studied adjuvant therapy. TH which requires trained personnel, MgSO4 being cost-effective and easily administered especially in resource-limited settings discriminate it from other potentials. Elucidated by Randomized Clinical Trials, TH combined with MgSO4 showed significantly better short-term outcomes than TH alone or supportive care, including shorter mechanical ventilation and respiratory support duration, reduced seizure frequency, earlier feeding initiation, and less reliance on antiepileptic medications. 58 This synergism may be attributed to the stability of plasma membrane and antagonism of N-methyl-D-aspartate (NMDA) Glutamate receptors achieved by MgSO4, which manifest as less MRI-detected brain damage. 59 For instance, the inhibition of this excitotoxic damage mitigates secondary neuronal injury, such as cell swelling, free radical production, and apoptosis, which are common in hypoxic-ischemic events. 60 Given that promising adjuvant therapy, long-term research is fundamental to legitimize its neurodevelopmental benefits and to overcome the challenge of achieving effective cerebrospinal fluid concentrations and ensuring timely intervention. Moreover, studies have investigated its use as a monotherapy in the management of HIE in preterm infants (<35 weeks of gestation) who are not eligible for therapeutic hypothermia and it has shown promising results in reducing cerebral palsy in these vulnerable populations. 61 One pilot study evaluated the safety and feasibility of using MgSO4 in combination with EPO and TH in neonates and concluded that such a combination is feasible as no patient experienced any serious adverse events or death. 18 However, this is a low level of evidence as the sample size was small (only 9 neonates were included), there was no control group, and lastly the study was not blinded. long-term improvements in neurodevelopment offered by MgSO4 remains a riddle for many ongoing trials and call for further research exploring therapeutic adjuvants for this population.
3. Melatonin
Melatonin was also one of the promising therapies discussed in the literature for the management of neonates with HIE. Melatonin was reported to have a neuroprotective effect based on studies done on piglet and animal models, as it was found to act as an excitotoxicity inhibitor, prevent cell death, reduce inflammation, and work as an antioxidant. 62–64 There are strong preclinical studies, including meta- analyses, showing the positive effects of the use of melatonin in neonate animals with HIE and as an adjuvant therapy with TH. 62,63,65 Moreover, a systemic review of the few limited clinical trials on the effect of melatonin as a mono- or adjunct therapy with TH in neonates with HIE reported promising evidence as it was found to be associated with lower risk of seizure, white matter injury, mortality rate, and neurodevelopmental impairment. 66,67 These findings, along with melatonin’s favorable safety profile, encourage larger and more advanced clinical trials to provide stronger evidence of the efficacy of melatonin in the management of neonates with HIE. In addition, in vivo study on the effect of the double therapy of melatonin with EPO in HIE animal neonates model showed promising findings as this combination was associated with better EEG findings and oligodendrocyte lifespan and survival. 68 Therefore, clinical studies are needed to investigate the efficacy of this combination in neonates with HIE.
4. Xenon
Gases play a crucial role in the medical field, ever since their introduction into the world of anesthesia where they showed anesthetic and analgesic properties, the era of gas application in the therapeutic industries expanded. Noble gases such as xenon and argon are the main pillars of many ongoing research exploring their potential as adjuvants in enhancing the neurodevelopmental outcomes in HIE. The unique properties of these nobles made their synergism with hypothermia promising. Xenon with its rapid onset NMDA receptor antagonist, preventing glutamate-induced excitotoxicity, easy titratability, and ability to cross the blood-brain barrier without increasing intracranial pressure or causing fetal toxicity reflecting safe use in children, being administered early on showed promising outcomes in reducing seizures associated with HIE. 19,69,70 Limitations of xenon to be explored with future research to optimize its administration, revolve around its scarcity, high costs, and the need for specialized delivery systems, restricting its efficacy as an adjunct to TH, as shown by the TOBY- Xe trial. 19,69 Argon on the other hand, its availability, lower cost, and ability to block GABA receptors succeed in neuroprotection pre-clinical tests, 70 yet to be translated into clinical studies.
Synthetic analogs therapy and stem cells
The multifarious approach adopted in the management of HIE consists of minimizing inflammation, oxidative stress, and apoptosis to improve outcomes in neonates with HIE. Sovateltide - endothelin-B synthetic analog - has gone through Clinical trials (phases I, II, and III) for treating acute cerebral ischemic stroke which has a similar mechanism of injury exhibited by HIE. 17Trails have proven their effectiveness in improving neurological outcomes explained by increased expression of endothelin-B receptors, vascular endothelial growth factor, and nerve growth factor which are key mediators in neuronal development, differentiation, proliferation, and migration. 17 All in all, Sovateltide mechanisms of action address the primary and secondary energy failure by enhancing hypoxia-induced survival factors, providing a compelling basis for exploring its potential in HIE therapy. 17 Currently, with the expansion of stem cell scope, few studies have examined the effect of autologous umbilical cord blood (UCB) on neurological functional improvement with some positive results. 71,72 A Korean study involving 96 children between the ages of 10 months and 10 years demonstrated noteworthy improvements in those receiving allogeneic UCB cells along with erythropoietin compared to ones who received erythropoietin alone or placebo. 72 Given these encouraging results of stem cell therapy, investigating their role in treating HIE a common cause of cerebral palsy is appealing with much more to explore including dose-ranging, optimal administration route and long term follow up.
The need for further studies of therapeutic hypothermia
There are several reasons to conduct additional research on hypothermia. Despite TH improving the outcome of death and disability significantly, 46% of infants who received this intervention either died or were found to have moderate to severe disabilities. [2]
Although this number varies in different trials, deleterious outcomes are still serious. So, there is still much chance for improvement in outcomes. Since hypothermia was tested in a particular subset of newborns, further investigations are required to address other groups of newborns with HIE like preterm infants less than 35-week GA and those with mild encephalopathy, in addition to investigating neuroprotection with adjunctive therapies.
TH represents a significant key advancement in the management of neonatal HIE. However, many challenges are still encountered in clinical practice. Despite promising current results, the negative outcomes of HIE are still a heavy burden for families and medical staff. Although several agents being investigated in combination with TH, the results need to be confirmed with further research and cannot be currently adopted as standard of care. Ongoing research and equitable access to this therapy are critical for further improving outcomes in affected neonates. Precision medicine could play a role in future HIE management. The integration of novel treatments, therapy approaches, and long-term follow-up studies will be essential in advancing this field.
Authors’ contribution
All authors have significantly contributed to the work, whether by conducting literature searches, drafting, revising, or critically reviewing the article. They have given their final approval of the version to be published, have agreed with the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Conflict of interest
None.
Source of funding
None.
References
1. Korf JM, Mccullough LD, Caretti V. A narrative review on treatment strategies for neonatal hypoxic ischemic encephalopathy. Transl Pediatr. 2023;12(8):1552–71.
2. Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;31(1):CD003311.
3. Acun C, Karnati S, Padiyar S, Puthuraya S, Aly H, Mohamed M. Trends of neonatal hypoxic-ischemic encephalopathy prevalence and associated risk factors in the United States. Am J Obstet Gynecol. 2010;275(5):751.
4. Kurinczuk JJ, Koning MW, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329–38.
5. Andersen M, Andelius TCK, Pedersen MV, Kyng KJ, Henriksen TB. Severity of hypoxic ischemic encephalopathy and heart rate variability in neonates: a systematic review. BMC Pediatr. 2019;19(1):242.
6. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705.
7. Power BD, Mcginley J, Sweetman D, Murphy JFA. The Modified Sarnat Score in the Assessment of Neonatal Encephalopathy: A Quality Improvement Initiative. Ir Med J. 2019;112(7):976.
8. Thompson CM, Puterman AS, Linley LL, Hann FM, Van Der Elst C, Molteno CD, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757–61.
9. Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558–66.
10. Mathew JL, Kaur N, Dsouza JM. Therapeutic hypothermia in neonatal hypoxic encephalopathy: A systematic review and meta-analysis. J Glob Health. 2022;12:4030.
11. Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–70.
12. Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, Mcdonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84.
13. Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58.
14. Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126(4):771–8.
15. Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, Mcnamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(8):692–700.
16. Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, Zhuang DY, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157(3):367–72.
17. Ranjan AK, Gulati A. Advances in Therapies to Treat Neonatal Hypoxic-Ischemic Encephalopathy. J Clin Med. 2023;20:6653.
18. Nonomura M, Harada S, Asada Y, Matsumura H, Iwami H, Tanaka Y, et al. Combination therapy with erythropoietin, magnesium sulfate and hypothermia for hypoxic-ischemic encephalopathy: an open- label pilot study to assess the safety and feasibility. BMC Pediatr. 2019;19(1):13.
19. Victor S, Ferreira ER, Rahim A, Hagberg H, Edwards D. New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy. Eur J Pediatr. 2022;181‘(3):875–87.
20. Lemyre B, Chau V. Hypothermia for newborns with hypoxic-ischemic encephalopathy. Paediatr Child Health. 2018;23:285–91.
21. Drury PP, Gunn ER, Bennet L, Gunn AJ. Mechanisms of hypothermic neuroprotection. Clin Perinatol. 2014;41(1):161–75.
22. Wassink G, Lear CA, Gunn KC, Dean JM, Bennet L, Gunn AJ. Analgesics, sedatives, anticonvulsant drugs, and the cooled brain. Semin Fetal Neonatal Med. 2015;20(2):109–14.
23. Papile LA, Baley JE, Benitz W, Cummings J, Carlo WA, Eichenwald E, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146–50.
24. Akula VP, Joe P, Thusu K, Davis AS, Tamaresis JS, Kim S, et al. A randomized clinical trial of therapeutic hypothermia mode during transport for neonatal encephalopathy. J Pediatr. 2015;166(4):856– 61.
25. Pfister RH, Bingham P, Edwards EM, Horbar JD, Kenny MJ, Inder T, et al. The Vermont Oxford Neonatal Encephalopathy Registry: rationale, methods, and initial results. BMC Pediatr. 2012;12:84.
26. Lemyre B, Ly L, Chau V, Chacko A, Barrowman N, Whyte H, et al. Initiation of passive cooling at referring centre is most predictive of achieving early therapeutic hypothermia in asphyxiated newborns. Paediatr Child Health. 2017;22(5):264–8.
27. Strohm B, Hobson A, Brocklehurst P, Edwards AD, Azzopardi D. Subcutaneo
